
Bioprocess Engineering 2nd Edition by Fikret Kargi,Michael Shuler
Edition 2ISBN: 9780130819086
Bioprocess Engineering 2nd Edition by Fikret Kargi,Michael Shuler
Edition 2ISBN: 9780130819086 Exercise 7
Components A and B of a binary mixture are to be separated in a chromatographic column.
The adsorption isotherms of these compounds are given by the following equations: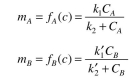 where
where 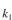 = 0.2 mg solute A absorbed/mg adsorbent
= 0.2 mg solute A absorbed/mg adsorbent 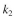 = 0.1 mg solute/ml liquid
= 0.1 mg solute/ml liquid 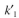 = 0.05 mg solute B adsorbed/mg adsorbent
= 0.05 mg solute B adsorbed/mg adsorbent 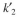 = 0.02 mg solute/ml liquid
= 0.02 mg solute/ml liquid
The bed contains 3 g of very fine support particles. The bed volume is 150 ml, bed porosity is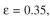 and the cross-sectional area of the bed is
and the cross-sectional area of the bed is 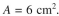 If the volume of the mixture
If the volume of the mixture
added is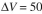 ml, determine the following:
ml, determine the following:
a. Position of each band A and B in the column,
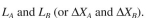
b.
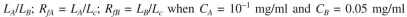 in liquid
in liquid
phase at equilibrium.
The adsorption isotherms of these compounds are given by the following equations:
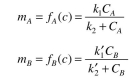 where
where 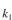 = 0.2 mg solute A absorbed/mg adsorbent
= 0.2 mg solute A absorbed/mg adsorbent 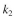 = 0.1 mg solute/ml liquid
= 0.1 mg solute/ml liquid 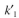 = 0.05 mg solute B adsorbed/mg adsorbent
= 0.05 mg solute B adsorbed/mg adsorbent 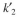 = 0.02 mg solute/ml liquid
= 0.02 mg solute/ml liquidThe bed contains 3 g of very fine support particles. The bed volume is 150 ml, bed porosity is
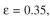 and the cross-sectional area of the bed is
and the cross-sectional area of the bed is 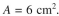 If the volume of the mixture
If the volume of the mixtureadded is
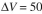 ml, determine the following:
ml, determine the following: a. Position of each band A and B in the column,
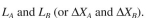
b.
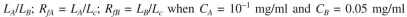 in liquid
in liquidphase at equilibrium.
Explanation
(a)Components A and B of a binary mixtur...
Bioprocess Engineering 2nd Edition by Fikret Kargi,Michael Shuler
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255


