Multiple Choice
How many lone pairs of electrons need to be added to the Lewis structure of carbonic acid shown below? 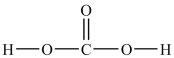
A) 0
B) 3
C) 4
D) 6
E) 7
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q16: A resonance hybrid is a composite of
Q71: The correct name for SF<sub>6</sub> is sulfur
Q77: The Lewis structure of formaldehyde is shown
Q78: The Lewis structure for the molecule below
Q79: What is the molecular shape around the
Q80: The molecule below is a polar molecule.
Q81: The covalent bond between chlorine and
Q83: Aspartic acid is an amino acid used
Q85: Which bond is the least polar?<br>A)C-N<br>B)C-O<br>C)C-C<br>D)C-Cl<br>E)C-F
Q86: A double bond is counted as two