Deck 2: The Chemical Level of Organization
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/105
Play
Full screen (f)
Deck 2: The Chemical Level of Organization
1
Which of the following elements is found in all organic molecules?
A) nitrogen
B) oxygen
C) iron
D) carbon
E) copper
A) nitrogen
B) oxygen
C) iron
D) carbon
E) copper
D
2
A(n) ________ contains atoms with the same atomic number.
A) base
B) element
C) cation
D) anion
E) enzyme
A) base
B) element
C) cation
D) anion
E) enzyme
B
3
The bond between sodium and chlorine is a(n) ________ bond.
A) covalent
B) ionic
C) hydrogen
D) hydrophobic
E) metallic
A) covalent
B) ionic
C) hydrogen
D) hydrophobic
E) metallic
B
4
How many electrons do most atoms need in their second outer shell in order to be stable?
A) two
B) three
C) four
D) six
E) eight
A) two
B) three
C) four
D) six
E) eight

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
5
Which statement is true regarding atomic structure and function?
A) Electrons and protons comprise the nucleus of the atom.
B) An atom is electrically neutral, having the same number of positively charged protons and negatively charged electrons.
C) Neutrons are found in orbits around the atomic nucleus.
D) The charge of an atom depends on where the protons and electrons are located.
E) All shells around the nucleus optimally contain 8 electrons.
A) Electrons and protons comprise the nucleus of the atom.
B) An atom is electrically neutral, having the same number of positively charged protons and negatively charged electrons.
C) Neutrons are found in orbits around the atomic nucleus.
D) The charge of an atom depends on where the protons and electrons are located.
E) All shells around the nucleus optimally contain 8 electrons.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
6
An oxygen atom can have 8, 9, or 10 neutrons in its nucleus. This variation describes
A) atomic number.
B) electric charge.
C) bonding characteristics.
D) isotopes.
E) proton therapy.
A) atomic number.
B) electric charge.
C) bonding characteristics.
D) isotopes.
E) proton therapy.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
7
The atomic number of an atom is determined by the
A) number of protons.
B) number of neutrons.
C) number and arrangement of electrons.
D) size of the atom.
E) mass of the atom.
A) number of protons.
B) number of neutrons.
C) number and arrangement of electrons.
D) size of the atom.
E) mass of the atom.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
8
Atoms that are of the same element but contain different numbers of neutrons are called
A) isomers.
B) cations.
C) isotopes.
D) anions.
E) None of these is correct.
A) isomers.
B) cations.
C) isotopes.
D) anions.
E) None of these is correct.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
9
The uncharged subatomic particles are called
A) atoms.
B) molecules.
C) protons.
D) neutrons.
E) electrons.
A) atoms.
B) molecules.
C) protons.
D) neutrons.
E) electrons.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
10
Combinations of atoms that contain two or more different elements are called
A) molecules.
B) compounds.
C) mixtures.
D) isotopes.
E) None of these is correct.
A) molecules.
B) compounds.
C) mixtures.
D) isotopes.
E) None of these is correct.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
11
The mass number represents the number of
A) protons in an atom.
B) electrons in an ion.
C) neutrons in an atom.
D) protons and neutrons in an atom.
E) neutrons and electrons in an atom.
A) protons in an atom.
B) electrons in an ion.
C) neutrons in an atom.
D) protons and neutrons in an atom.
E) neutrons and electrons in an atom.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
12
The area around the center of an atom, which contains negatively charged subatomic particles, is called the electron
A) cloud.
B) nucleus.
C) active site.
D) buffering zone.
E) double helix.
A) cloud.
B) nucleus.
C) active site.
D) buffering zone.
E) double helix.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
13
Ions with a negative charge are called
A) cations.
B) anions.
C) radicals.
D) polyatomic ions.
E) None of these is correct.
A) cations.
B) anions.
C) radicals.
D) polyatomic ions.
E) None of these is correct.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
14
If an element is composed of atoms with an atomic number of 8 and a mass number of 14, then an electrically neutral atom of this element contains
A) 6 protons.
B) 6 neutrons.
C) 6 electrons.
D) 14 protons.
E) 14 electrons.
A) 6 protons.
B) 6 neutrons.
C) 6 electrons.
D) 14 protons.
E) 14 electrons.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
15
An element has 6 electrons, 6 protons, and 6 neutrons. What will be the mass number of that element?
A) 6
B) 12
C) 18
D) 36
E) 15
A) 6
B) 12
C) 18
D) 36
E) 15

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
16
Covalent bonds are formed when
A) atoms share electrons.
B) cations and anions are held together by their opposite charges.
C) electrons are exchanged between atoms.
D) hydrogen forms bonds with negatively charged atoms in the same or different molecules.
E) two or more atoms lose electrons at the same time.
A) atoms share electrons.
B) cations and anions are held together by their opposite charges.
C) electrons are exchanged between atoms.
D) hydrogen forms bonds with negatively charged atoms in the same or different molecules.
E) two or more atoms lose electrons at the same time.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following would have a negative charge?
A) an atom
B) a molecule
C) a proton
D) a neutron
E) an electron
A) an atom
B) a molecule
C) a proton
D) a neutron
E) an electron

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
18
A molecule containing two atoms of hydrogen and one atom of oxygen in combination is called a(n)
A) oxygen molecule.
B) carbon dioxide molecule.
C) water molecule.
D) hydroxyl molecule.
E) hydroxide molecule.
A) oxygen molecule.
B) carbon dioxide molecule.
C) water molecule.
D) hydroxyl molecule.
E) hydroxide molecule.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
19
Which is the correct description of a molecule?
A) It is an electrically charged atom.
B) It cannot be broken down physically.
C) It is comprised of two or more elements bonded together.
D) It is the smallest unit of matter.
E) It is comprised of two or more atoms sharing electrons.
A) It is an electrically charged atom.
B) It cannot be broken down physically.
C) It is comprised of two or more elements bonded together.
D) It is the smallest unit of matter.
E) It is comprised of two or more atoms sharing electrons.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is sometimes used in diagnostic imaging?
A) a radioisotope
B) a proton
C) an ion
D) an atom
E) an electrolyte
A) a radioisotope
B) a proton
C) an ion
D) an atom
E) an electrolyte

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
21
In a molecule of hydrogen, a pair of electrons is shared equally. Such a bond is called a(n)
A) ionic bond.
B) polar covalent bond.
C) nonpolar covalent bond.
D) oxygen covalent bond.
E) hydrogen bond.
A) ionic bond.
B) polar covalent bond.
C) nonpolar covalent bond.
D) oxygen covalent bond.
E) hydrogen bond.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
22
If two pairs of electrons are shared between two atoms, what type of bond occurs?
A) single covalent bond
B) double covalent bond
C) triple covalent bond
D) polar covalent bond
E) hydrogen bond
A) single covalent bond
B) double covalent bond
C) triple covalent bond
D) polar covalent bond
E) hydrogen bond

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is an example of an anion?
A) magnesium
B) potassium
C) calcium
D) chloride
E) sodium
A) magnesium
B) potassium
C) calcium
D) chloride
E) sodium

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
24
The symbol ⇌, used in visual chemical reactions, means that
A) the chemical reaction can go in either direction.
B) the concentration of the end products is the same as that of the reactants.
C) the substrates can become reactants and vice versa.
D) there is more end product than reactant.
E) input of energy is required.
A) the chemical reaction can go in either direction.
B) the concentration of the end products is the same as that of the reactants.
C) the substrates can become reactants and vice versa.
D) there is more end product than reactant.
E) input of energy is required.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
25
The reaction NaOH + HCl → NaCl + H2O would be an example of a(n)
A) exchange reaction.
B) decomposition reaction.
C) synthesis reaction.
D) enzyme reaction.
E) metabolic reaction.
A) exchange reaction.
B) decomposition reaction.
C) synthesis reaction.
D) enzyme reaction.
E) metabolic reaction.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
26
Reactions that ultimately result in larger molecules formed from smaller ones are called ________ reactions.
A) hydrolysis
B) reversible
C) exergonic
D) dissociation
E) synthesis
A) hydrolysis
B) reversible
C) exergonic
D) dissociation
E) synthesis

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
27
In an endergonic reaction,
A) large molecules are broken down into smaller ones.
B) small molecules are assembled into larger ones.
C) molecules are rearranged to form new molecules.
D) molecules move from reactants to products and back.
E) energy is consumed during the reaction.
A) large molecules are broken down into smaller ones.
B) small molecules are assembled into larger ones.
C) molecules are rearranged to form new molecules.
D) molecules move from reactants to products and back.
E) energy is consumed during the reaction.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
28
Hydrolysis is an example of which type of reaction?
A) exchange
B) reversible
C) anabolism
D) synthesis
E) decomposition
A) exchange
B) reversible
C) anabolism
D) synthesis
E) decomposition

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
29
The term that applies to all of the decomposition reactions that occur in metabolism is
A) anabolism.
B) dehydration synthesis.
C) catabolism.
D) ionization.
E) homeostasis.
A) anabolism.
B) dehydration synthesis.
C) catabolism.
D) ionization.
E) homeostasis.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is a weak electrical attraction between molecules?
A) ionic bond
B) covalent bond
C) polar bond
D) metallic bond
E) hydrogen bond
A) ionic bond
B) covalent bond
C) polar bond
D) metallic bond
E) hydrogen bond

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
31
Ions with a positive charge are called
A) anions.
B) bases.
C) metabolites.
D) cations.
E) acids.
A) anions.
B) bases.
C) metabolites.
D) cations.
E) acids.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
32
Which statement about the reaction H2 + Cl2 → 2HCl is correct?
A) H2 and Cl2 are the products.
B) HCl is the product.
C) One molecule of hydrogen contains one atom.
D) One molecule of chlorine contains one atom.
E) The reaction is unbalanced.
A) H2 and Cl2 are the products.
B) HCl is the product.
C) One molecule of hydrogen contains one atom.
D) One molecule of chlorine contains one atom.
E) The reaction is unbalanced.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
33
The addition of energy to start a reaction is called the energy of
A) endergonic control.
B) activation.
C) exergonic control.
D) release.
E) equilibrium.
A) endergonic control.
B) activation.
C) exergonic control.
D) release.
E) equilibrium.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
34
Which of these is a molecule but is not considered to be a compound?
A) H2O
B) CO2
C) C6H12O6
D) H2
E) CO
A) H2O
B) CO2
C) C6H12O6
D) H2
E) CO

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
35
Chlorine atoms have seven electrons in the outermost shell. As a result, one would expect chlorine to form ions with a charge of
A) +1.
B) +2.
C) 0.
D) -2.
E) -1.
A) +1.
B) +2.
C) 0.
D) -2.
E) -1.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
36
Chemical reactions that occur in the human body are catalyzed by special protein molecules called
A) electrolytes.
B) enzymes.
C) metabolites.
D) steroids.
E) buffers.
A) electrolytes.
B) enzymes.
C) metabolites.
D) steroids.
E) buffers.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is a characteristic of hydrogen bonds?
A) Hydrogen bonds are strong attractive forces between hydrogen atoms and negatively charged atoms.
B) Hydrogen bonds occur only in water.
C) Hydrogen bonds can form between adjacent molecules.
D) Hydrogen bonds are part of fatty-acid structure.
E) Hydrogen bonds are part of carbohydrate structure.
A) Hydrogen bonds are strong attractive forces between hydrogen atoms and negatively charged atoms.
B) Hydrogen bonds occur only in water.
C) Hydrogen bonds can form between adjacent molecules.
D) Hydrogen bonds are part of fatty-acid structure.
E) Hydrogen bonds are part of carbohydrate structure.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
38
Choose the most accurate definition of chemical reaction.
A) It is a process in which bonds between atoms are formed or broken.
B) It is the energy of motion.
C) It is an increase in random molecular motion.
D) It is movement or a change in the physical structure of matter.
E) It is the capacity to perform work.
A) It is a process in which bonds between atoms are formed or broken.
B) It is the energy of motion.
C) It is an increase in random molecular motion.
D) It is movement or a change in the physical structure of matter.
E) It is the capacity to perform work.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
39
Chemical reactions that release energy are categorized as
A) endergonic.
B) catabolic.
C) anabolic.
D) hydrolytic.
E) exergonic.
A) endergonic.
B) catabolic.
C) anabolic.
D) hydrolytic.
E) exergonic.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
40
When two monosaccharides undergo a dehydration synthesis,
A) a new monosaccharide is formed.
B) a starch is formed.
C) a polysaccharide is formed.
D) a condensation reaction occurs.
E) hydrolysis occurs.
A) a new monosaccharide is formed.
B) a starch is formed.
C) a polysaccharide is formed.
D) a condensation reaction occurs.
E) hydrolysis occurs.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
41
Which is the mechanism of enzyme functioning?
A) Enzymes raise the activation energy of a reaction.
B) Enzymes remove hydrogen ions.
C) Enzymes lower the activation energy of a reaction.
D) Enzymes replace hydrogen ions.
E) Enzymes promote complementary base-pairing.
A) Enzymes raise the activation energy of a reaction.
B) Enzymes remove hydrogen ions.
C) Enzymes lower the activation energy of a reaction.
D) Enzymes replace hydrogen ions.
E) Enzymes promote complementary base-pairing.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following are defined as soluble inorganic compounds whose ions will conduct an electric current in solutions?
A) catalysts
B) electrolytes
C) strong acids
D) buffers
E) steroid hormones
A) catalysts
B) electrolytes
C) strong acids
D) buffers
E) steroid hormones

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following pairs of elements can be classified as inorganic only?
A) sodium and hydrogen
B) carbon and oxygen
C) calcium and carbon
D) hydrogen and carbon
E) sodium and calcium
A) sodium and hydrogen
B) carbon and oxygen
C) calcium and carbon
D) hydrogen and carbon
E) sodium and calcium

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
44
A buffer
A) removes or replaces hydrogen ions in a solution.
B) is a compound with an extra electron in its outer shell.
C) has an unstable nucleus, making it highly reactive.
D) donates hydrogen ions to a solution.
E) consists of long carbon-carbon chains.
A) removes or replaces hydrogen ions in a solution.
B) is a compound with an extra electron in its outer shell.
C) has an unstable nucleus, making it highly reactive.
D) donates hydrogen ions to a solution.
E) consists of long carbon-carbon chains.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
45
________ are compounds that maintain the pH of solutions within given limits.
A) Enzymes
B) Electrolytes
C) Metabolites
D) Isotopes
E) Buffers
A) Enzymes
B) Electrolytes
C) Metabolites
D) Isotopes
E) Buffers

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following is an example of a strong base?
A) NaCl
B) NaOH
C) HCl
D) KF
E) H2O
A) NaCl
B) NaOH
C) HCl
D) KF
E) H2O

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
47
Water is an excellent solvent because
A) it dissolves all solutes.
B) water molecules covalently bond with atoms in other molecules.
C) it has a high heat capacity.
D) it makes up a major part of every cell.
E) water molecules are polar.
A) it dissolves all solutes.
B) water molecules covalently bond with atoms in other molecules.
C) it has a high heat capacity.
D) it makes up a major part of every cell.
E) water molecules are polar.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
48
Carbon dioxide is produced as a result of
A) metabolic activity.
B) carbon monoxide instability.
C) oxygen synthesis during aerobic respiration.
D) the breakdown of water into oxygen.
E) glucose synthesis.
A) metabolic activity.
B) carbon monoxide instability.
C) oxygen synthesis during aerobic respiration.
D) the breakdown of water into oxygen.
E) glucose synthesis.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
49
If a substance resists changes in pH, either by removing or replacing hydrogen ions, it is called
A) neutral.
B) acidic.
C) alkaline.
D) a buffer.
E) a salt.
A) neutral.
B) acidic.
C) alkaline.
D) a buffer.
E) a salt.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following constitutes most of the total body weight in humans?
A) water
B) acids
C) bases
D) salts
E) organic molecules
A) water
B) acids
C) bases
D) salts
E) organic molecules

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following is inorganic?
A) fatty acid
B) protein
C) DNA
D) sodium
E) glycogen
A) fatty acid
B) protein
C) DNA
D) sodium
E) glycogen

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
52
Why is it important to precisely regulate the pH of blood or other body fluids?
A) Blood functions as an excellent solvent.
B) Blood and other body fluids have a very high heat capacity.
C) Dehydration synthesis of large molecules occurs.
D) Hydrogen ions are extremely reactive.
E) Some organic molecules have polar covalent bonds.
A) Blood functions as an excellent solvent.
B) Blood and other body fluids have a very high heat capacity.
C) Dehydration synthesis of large molecules occurs.
D) Hydrogen ions are extremely reactive.
E) Some organic molecules have polar covalent bonds.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
53
During ionization, water molecules disrupt the ionic bonds of a solute, resulting in a mixture of ions that can conduct an electrical current in solution. These ions are called
A) cations.
B) anions.
C) isotopes.
D) electrolytes.
E) reactants.
A) cations.
B) anions.
C) isotopes.
D) electrolytes.
E) reactants.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following substances would be nearest the pH of human blood?
A) milk, pH ≈ 6.5
B) pure water, pH ≈ 7
C) tomato juice, pH ≈ 4
D) wine, pH ≈ 3
E) stomach secretions, pH ≈ 1
A) milk, pH ≈ 6.5
B) pure water, pH ≈ 7
C) tomato juice, pH ≈ 4
D) wine, pH ≈ 3
E) stomach secretions, pH ≈ 1

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
55
The best definition of organic material is anything that contains
A) carbon and oxygen covalently bonded.
B) carbon, oxygen, and hydrogen covalently bonded.
C) carbon and hydrogen covalently bonded.
D) hydrogen covalently bonded.
E) carbon, nitrogen, and hydrogen covalently bonded.
A) carbon and oxygen covalently bonded.
B) carbon, oxygen, and hydrogen covalently bonded.
C) carbon and hydrogen covalently bonded.
D) hydrogen covalently bonded.
E) carbon, nitrogen, and hydrogen covalently bonded.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following is an organic compound?
A) CO2
B) CH4
C) H2O
D) CCl4
E) HCl
A) CO2
B) CH4
C) H2O
D) CCl4
E) HCl

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
57
A solution containing more hydrogen ions than hydroxide ions is
A) acidic.
B) basic.
C) neutral.
D) alkaline.
E) organic.
A) acidic.
B) basic.
C) neutral.
D) alkaline.
E) organic.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
58
A mixture of water and a salt would result in breaking down the salt into a mixture of cations and anions. This process is called
A) dehydration synthesis.
B) dissociation.
C) hydrolysis.
D) condensation reaction.
E) equilibrium.
A) dehydration synthesis.
B) dissociation.
C) hydrolysis.
D) condensation reaction.
E) equilibrium.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
59
What is the primary composition of organic compounds?
A) carbon and oxygen atoms
B) oxygen and hydrogen atoms
C) oxygen and nitrogen atoms
D) carbon and hydrogen atoms
E) nitrogen and carbon atoms
A) carbon and oxygen atoms
B) oxygen and hydrogen atoms
C) oxygen and nitrogen atoms
D) carbon and hydrogen atoms
E) nitrogen and carbon atoms

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
60
The most acidic solution would have a pH of
A) 0.
B) 7.
C) 14.
D) 4.
E) 10.
A) 0.
B) 7.
C) 14.
D) 4.
E) 10.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
61
Which carbohydrate and example pair are not correctly matched?
A) glycogen - monosaccharide
B) glucose - monosaccharide
C) starch - polysaccharide
D) fructose - monosaccharide
E) sucrose - disaccharide
A) glycogen - monosaccharide
B) glucose - monosaccharide
C) starch - polysaccharide
D) fructose - monosaccharide
E) sucrose - disaccharide

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
62
 Figure 2-2 A Molecule
Figure 2-2 A MoleculeUse Figure 2-2 to answer the following question:
The molecule shown in the figure is considered to be the most important metabolic "fuel" in the body. Choose the best category of molecules to which it belongs.
A) steroid
B) saturated fatty acid
C) monoglyceride
D) cholesterol
E) monosaccharide

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
63
Enzymes
A) are lipids.
B) function as biological catalysts.
C) raise the activation energy for a reaction.
D) are carbohydrates.
E) are derived from cholesterol.
A) are lipids.
B) function as biological catalysts.
C) raise the activation energy for a reaction.
D) are carbohydrates.
E) are derived from cholesterol.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
64
A class of lipids used as chemical messengers, to signal cells to undergo changes, is called
A) polysaccharides.
B) phospholipids.
C) triglycerides.
D) steroids.
E) monoglycerides.
A) polysaccharides.
B) phospholipids.
C) triglycerides.
D) steroids.
E) monoglycerides.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
65
You are working with a particular protein in your research lab to determine how its properties affect the function of the protein. To determine what consequences there might be on protein function, you decide to change just one amino acid of the entire length of the protein, which consists of a total of 100 amino acids. You have just directly changed the ________ structure of the protein.
A) primary
B) secondary
C) tertiary
D) quaternary
E) binary
A) primary
B) secondary
C) tertiary
D) quaternary
E) binary

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
66
Lipids are used for which of the following?
A) to form essential structural components of cells
B) to provide roughly 10 times as much energy as carbohydrates
C) to help reduce body temperature
D) to help protect the skeleton
E) to carry genetic information
A) to form essential structural components of cells
B) to provide roughly 10 times as much energy as carbohydrates
C) to help reduce body temperature
D) to help protect the skeleton
E) to carry genetic information

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
67
Some proteins, like hemoglobin or antibody molecules, require multiple polypeptide chains to come together to act as a single protein. If the association among the different polypeptide chains is blocked, the ________ structure of the protein is destroyed.
A) primary
B) secondary
C) tertiary
D) quaternary
E) binary
A) primary
B) secondary
C) tertiary
D) quaternary
E) binary

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
68
The most important metabolic fuel molecule in the body is
A) sucrose.
B) starch.
C) protein.
D) vitamin B12.
E) glucose.
A) sucrose.
B) starch.
C) protein.
D) vitamin B12.
E) glucose.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
69
Amino acids contain a central carbon atom adjacent to a(n) ________ group and a(n) ________ group.
A) carboxyl; phosphate
B) nitrogenous; carboxyl
C) nitrogenous; amino
D) amino; carboxyl
E) amino; phosphate
A) carboxyl; phosphate
B) nitrogenous; carboxyl
C) nitrogenous; amino
D) amino; carboxyl
E) amino; phosphate

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following is an example of a disaccharide?
A) starch
B) glycogen
C) sucrose
D) cellulose
E) fructose
A) starch
B) glycogen
C) sucrose
D) cellulose
E) fructose

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
71
The group of organic compounds containing mostly carbon and hydrogen with small amounts of oxygen is defined as a
A) carbohydrate.
B) lipid.
C) protein.
D) nucleic acid.
E) fatty acid.
A) carbohydrate.
B) lipid.
C) protein.
D) nucleic acid.
E) fatty acid.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
72
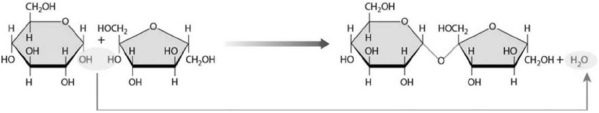 Figure 2-1 A Chemical Reaction
Figure 2-1 A Chemical ReactionUse Figure 2-1 to answer the following question:
Determine which reaction is shown in the figure and specify its mechanism of action.
A) The addition of a water molecule breaks down a complex molecule.
B) The removal of a water molecule breaks down a complex molecule.
C) Ionic bonds are broken apart as individual ions interact with the positive or negative ends of polar water molecules.
D) The removal of a water molecule facilitates the union of two molecules.
E) The addition of a water molecule facilitates the union of two molecules.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
73
Substrate molecules bind to enzymes at the
A) allosteric sites.
B) modification sites.
C) reaction sites.
D) active sites.
E) ionic sites.
A) allosteric sites.
B) modification sites.
C) reaction sites.
D) active sites.
E) ionic sites.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
74
Proteins are composed of units called
A) amino acids.
B) simple sugars.
C) fatty acids.
D) adenosines.
E) nucleotides.
A) amino acids.
B) simple sugars.
C) fatty acids.
D) adenosines.
E) nucleotides.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
75
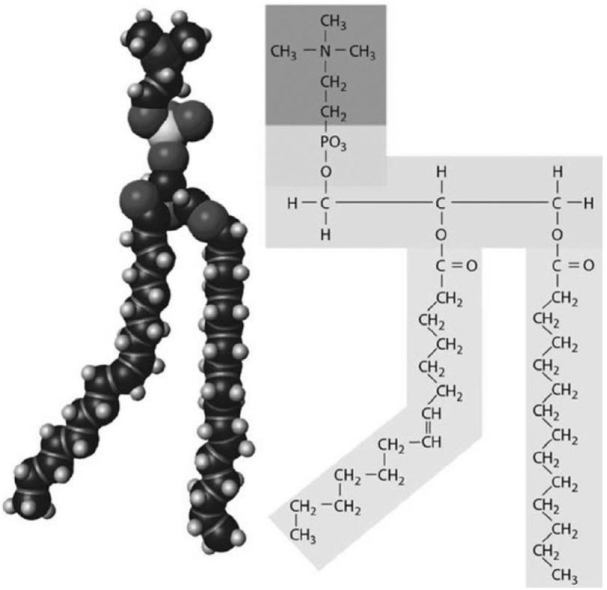 Figure 2-3 A Structure
Figure 2-3 A StructureUse Figure 2-3 to answer the following question:
Indicate the primary function(s) of the structure shown in the figure.
A) structural component of cell membranes
B) storage of glucose molecules
C) energy source
D) structural component of cell membranes, hormones, and digestive secretions in bile
E) energy source, energy storage, and insulation

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
76
A fatty acid that contains only single covalent bonds in its carbon chain is said to be
A) saturated.
B) polyunsaturated.
C) monounsaturated.
D) hydrogenated.
E) carboxylated.
A) saturated.
B) polyunsaturated.
C) monounsaturated.
D) hydrogenated.
E) carboxylated.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
77
Each amino acid forms bonds by connecting its carboxyl group to the next amino acid's
A) central carbon atom.
B) amino group.
C) carboxyl group.
D) hydroxyl group.
E) hydroxide group.
A) central carbon atom.
B) amino group.
C) carboxyl group.
D) hydroxyl group.
E) hydroxide group.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following can be denatured?
A) enzymes
B) ions
C) atoms
D) molecules
E) isotopes
A) enzymes
B) ions
C) atoms
D) molecules
E) isotopes

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
79
When placed in solution, an inorganic substance dissociates completely, forming hydrogen ions and anions. This substance would be a
A) strong base.
B) weak base.
C) strong acid.
D) weak acid.
E) salt.
A) strong base.
B) weak base.
C) strong acid.
D) weak acid.
E) salt.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
80
Functionally, carbohydrates are most important as
A) storage of glucose molecules.
B) a part of nucleic acid structure.
C) sources of energy.
D) receptors of the cell surface.
E) insulation under the skin.
A) storage of glucose molecules.
B) a part of nucleic acid structure.
C) sources of energy.
D) receptors of the cell surface.
E) insulation under the skin.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck


