Deck 23: Transition Elements and Their Coordination Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/82
Play
Full screen (f)
Deck 23: Transition Elements and Their Coordination Compounds
1
If M represents a transition element, which of the following oxides should be the least basic?
A) MO
B)M2O
C)M2O3
D) MO2
E) MO3
A) MO
B)M2O
C)M2O3
D) MO2
E) MO3
MO3
2
A feature of transition metal chemistry is that these elements exhibit multiple oxidation states. Which one of the following elements exhibits the smallest number of different oxidation states?
A) Ti
B) Cr
C) Mn
D) Co
E) Zn
A) Ti
B) Cr
C) Mn
D) Co
E) Zn
Zn
3
Which of the following will be diamagnetic?
A) Ni2+
B) Cr2+
C) Mn2+
D) Co3+
E) Ti4+
A) Ni2+
B) Cr2+
C) Mn2+
D) Co3+
E) Ti4+
Ti4+
4
Which of the following ions is least likely to form colored compounds?
A) Mn2+
B) Cr5+
C) Sc3+
D) Fe3+
E) Co2+
A) Mn2+
B) Cr5+
C) Sc3+
D) Fe3+
E) Co2+

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
5
The most common oxidation state for ions of the transition elements is
A) +2.
B) +3.
C) +4.
D) +5.
E) +6.
A) +2.
B) +3.
C) +4.
D) +5.
E) +6.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
6
What is the highest possible oxidation state for molybdenum, Mo?
A) +2
B) +4
C) +6
D) +8
E) None of these choices are correct.
A) +2
B) +4
C) +6
D) +8
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following elements has the ground state electron configuration, [Xe]4f145d106s1?
A) Hg
B) Ag
C) Hf
D) Au
E) Th
A) Hg
B) Ag
C) Hf
D) Au
E) Th

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
8
The ground state electronic configuration of Zn2+ is
A)[Ar]4s23d8.
B)[Ar]4s23d10.
C)[Ar]4s13d9.
D)[Ar]3d10.
E)[Ar]3d8.
A)[Ar]4s23d8.
B)[Ar]4s23d10.
C)[Ar]4s13d9.
D)[Ar]3d10.
E)[Ar]3d8.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following will be paramagnetic?
A) V5+
B) Ni2+
C) Mn7+
D) Ti4+
E) Zn
A) V5+
B) Ni2+
C) Mn7+
D) Ti4+
E) Zn

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
10
Which one of the following has the ground state electron configuration [Ar]3d104s1?
A) In+
B) Cd2+
C) Ag+
D) Ag
E) Cu
A) In+
B) Cd2+
C) Ag+
D) Ag
E) Cu

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
11
What is the highest possible oxidation state for palladium, Pd?
A) +1
B) +2
C) +3
D) +4
E) +6
A) +1
B) +2
C) +3
D) +4
E) +6

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following transition elements can have an oxidation number of +7?
A) V
B) Cr
C) Mn
D) Fe
E) Co
A) V
B) Cr
C) Mn
D) Fe
E) Co

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
13
A certain transition element has the stable oxidation states of +2, +3, +4, +5, and +6. In which state the element be most likely to form an ionic bond with chlorine?
A) +2
B) +3
C) +4
D) +5
E) +6
A) +2
B) +3
C) +4
D) +5
E) +6

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
14
The most common oxidation state for ions of the inner transition elements is
A) +2.
B) +3.
C) +4.
D) +5.
E) +7.
A) +2.
B) +3.
C) +4.
D) +5.
E) +7.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following ions is most likely to form colored compounds?
A) Sc3+
B) Cu+
C) Zn2+
D) Cr3+
E) Ca2+
A) Sc3+
B) Cu+
C) Zn2+
D) Cr3+
E) Ca2+

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following atoms has the biggest radius?
A) Ti
B) Cr
C) Fe
D) Ni
E) Zn
A) Ti
B) Cr
C) Fe
D) Ni
E) Zn

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
17
A certain transition element has the stable oxidation states of +2, +3, +4, +5, and +6. In which state will the element be most likely to form a covalent bond with chlorine?
A) +2
B) +3
C) +4
D) +5
E) +6
A) +2
B) +3
C) +4
D) +5
E) +6

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
18
The ground state electronic configuration of Cr2+ is
A)[Ar]4s13d5.
B)[Ar]4s23d4.
C)[Ar]3d4.
D)[Ar]4s13d3.
E)[Ar]4s23d2.
A)[Ar]4s13d5.
B)[Ar]4s23d4.
C)[Ar]3d4.
D)[Ar]4s13d3.
E)[Ar]4s23d2.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
19
How many unpaired electrons are there in the Fe3+ ion?
A) 5
B) 4
C) 3
D) 2
E) 1
A) 5
B) 4
C) 3
D) 2
E) 1

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following transition elements can achieve the largest oxidation number?
A) chromium, Cr, Group 6B(6)
B)manganese, Mn, Group 7B(7)
C)iron, Fe, Group 8B(8)
D)cobalt, Co, Group 8B(9)
E)zinc, Zn, Group 2B(12)
A) chromium, Cr, Group 6B(6)
B)manganese, Mn, Group 7B(7)
C)iron, Fe, Group 8B(8)
D)cobalt, Co, Group 8B(9)
E)zinc, Zn, Group 2B(12)

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the oxidation states of chromium has the largest valence-state electronegativity?
A) chromium(0)
B)chromium(II)
C)chromium(III)
D)chromium(IV)
E)chromium(VI)
A) chromium(0)
B)chromium(II)
C)chromium(III)
D)chromium(IV)
E)chromium(VI)

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
22
The oxidation and coordination numbers of cobalt in the compound [Co(NH3)5Cl]Cl2 are,respectively
A) 2 and 6.
B) 2 and 8.
C) 3 and 6.
D) 3 and 8.
E) None of these choices are correct.
A) 2 and 6.
B) 2 and 8.
C) 3 and 6.
D) 3 and 8.
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
23
Chromium and manganese are among the transition elements that form several different oxides. Which of the following statements characterize these oxides?
A)As the oxidation number on the metal increases, the valence-state electronegativity increases and the oxides change from acidic to basic.
B)As the oxidation number on the metal increases, the valence-state electronegativity increases and the oxides change from basic to acidic.
C)As the oxidation number on the metal increases, the valence-state electronegativity decreases and the oxides change from acidic to basic.
D)As the oxidation number on the metal increases, the valence-state electronegativity decreases and the oxides change from basic to acidic.
E) None of these choices are correct.
A)As the oxidation number on the metal increases, the valence-state electronegativity increases and the oxides change from acidic to basic.
B)As the oxidation number on the metal increases, the valence-state electronegativity increases and the oxides change from basic to acidic.
C)As the oxidation number on the metal increases, the valence-state electronegativity decreases and the oxides change from acidic to basic.
D)As the oxidation number on the metal increases, the valence-state electronegativity decreases and the oxides change from basic to acidic.
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following will be the strongest oxidizing agent?
A) Cr
B) Cr(II)
C) Cr(III)
D) Cr(IV)
E) Cr(VI)
A) Cr
B) Cr(II)
C) Cr(III)
D) Cr(IV)
E) Cr(VI)

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
25
A characteristic of ligands is that
A) they are Lewis acids.
B)they are Lewis bases.
C)they are ions.
D)they are electron pair acceptors.
E)they are Brønsted-Lowry acids.
A) they are Lewis acids.
B)they are Lewis bases.
C)they are ions.
D)they are electron pair acceptors.
E)they are Brønsted-Lowry acids.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following coordination numbers applies to octahedral complexes?
A) 4
B) 5
C) 6
D) 8
E) None of these choices are correct.
A) 4
B) 5
C) 6
D) 8
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is considered a bidentate ligand?
A) cyanide, CN-
B) thiocyanate, SCN-
C)oxalate, C2O42-
D)nitrite, NO2-
E) hydroxide, OH-
A) cyanide, CN-
B) thiocyanate, SCN-
C)oxalate, C2O42-
D)nitrite, NO2-
E) hydroxide, OH-

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
28
The compound K3[Fe(CN)6] is used in calico printing and wool dyeing. Give its systematic name.
A) potassium iron(III) hexacyanate
B)tripotassium iron(III) hexacyanate
C)potassium hexacyanoferrate(III)
D)potassium hexacyanideferrate
E)None of these choices are correct.
A) potassium iron(III) hexacyanate
B)tripotassium iron(III) hexacyanate
C)potassium hexacyanoferrate(III)
D)potassium hexacyanideferrate
E)None of these choices are correct.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
29
A certain transition metal complex has the formula MX42+. If the metal ion has a d8 electron configuration, what is the shape of the complex?
A) octahedral
B)square pyramid
C)tetrahedral
D)trigonal pyramid
E)square planar
A) octahedral
B)square pyramid
C)tetrahedral
D)trigonal pyramid
E)square planar

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
30
When the ethylenediaminetetraacetate ion (EDTA4-) forms a complex with a transition metal ion, how many electrons does it normally donate to the metal?
A) 4
B) 6
C) 8
D) 10
E) 12
A) 4
B) 6
C) 8
D) 10
E) 12

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
31
In the compound [Ni(en)2(H2O)2]SO4 (where en = ethylenediamine) the oxidation number and coordination number of nickel are, respectively
A) 2 and 6.
B) 4 and 6.
C) 6 and 6.
D) 2 and 4.
E) 4 and 4.
A) 2 and 6.
B) 4 and 6.
C) 6 and 6.
D) 2 and 4.
E) 4 and 4.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
32
In the formation of a transition metal complex, the central metal atom or ion acts as
A) an Arrhenius acid.
B)a Bronsted-Lowry acid.
C)a Bronsted-Lowry base.
D)a Lewis acid.
E)a Lewis base.
A) an Arrhenius acid.
B)a Bronsted-Lowry acid.
C)a Bronsted-Lowry base.
D)a Lewis acid.
E)a Lewis base.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
33
In the compound K[Co(C2O4)2(H2O)2] (where C2O42- = oxalate) the oxidation number and coordination number of cobalt are, respectively
A) -1 and 4.
B) -1 and 6.
C) 3 and 4.
D) 3 and 6.
E) 1 and 6.
A) -1 and 4.
B) -1 and 6.
C) 3 and 4.
D) 3 and 6.
E) 1 and 6.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following should be the strongest reducing agent?
A) Fe
B) Ru
C) Os
D) Re
E) Cu
A) Fe
B) Ru
C) Os
D) Re
E) Cu

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
35
Aluminum reacts with oxygen in the air to form a protective oxide coating. Silver also reactswith compounds in air to form a black coating. What substance is formed?
A) silver oxide
B)silver chloride
C)silver sulfide
D)silver carbonate
E)silver nitride
A) silver oxide
B)silver chloride
C)silver sulfide
D)silver carbonate
E)silver nitride

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
36
Give the systematic name for [Cu(NH3)4]Cl2.
A) dichlorotetraamminecuprate(II)
B)tetraamminecopper(II) chloride
C)copper(II) ammonium chloride
D)tetraaminocopper(II) chloride
E)None of these choices are correct.
A) dichlorotetraamminecuprate(II)
B)tetraamminecopper(II) chloride
C)copper(II) ammonium chloride
D)tetraaminocopper(II) chloride
E)None of these choices are correct.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
37
Which one of the following normally acts as a bidentate ligand in complexes with transitionmetal ions?
A) CN-
B) EDTA4-
C) SCN-
D) ethylene diamine
E) ethylene, C2H4
A) CN-
B) EDTA4-
C) SCN-
D) ethylene diamine
E) ethylene, C2H4

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
38
Give the systematic name for Cr(CO)3(NH3)3.
A) chromiumtriaminotricarbonyl
B)triamminechromium carbonate
C)triamminetricarbonylchromate(0)
D)triamminetricarbonylchromium(0)
E)None of these choices are correct.
A) chromiumtriaminotricarbonyl
B)triamminechromium carbonate
C)triamminetricarbonylchromate(0)
D)triamminetricarbonylchromium(0)
E)None of these choices are correct.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
39
10.0 mL of a 0.100 mol/L solution of a metal ion M2+ is mixed with 10.0 mL of a 0.100 mol/L solution of a ligand L. A reaction occurs in which the product is ML3. Approximately, what is the maximum concentration of ML32+, in mol/L, which could result from this reaction?
A) 0.100
B) 0.050
C) 0.033
D) 0.025
E) 0.017
A) 0.100
B) 0.050
C) 0.033
D) 0.025
E) 0.017

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
40
What is the coordination number of cobalt in the complex ion [Co(en)Cl4]-? (en = ethylenediamine)
A) 1
B) 2
C) 4
D) 6
E) 8
A) 1
B) 2
C) 4
D) 6
E) 8

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following octahedral complexes should have the largest crystal field splitting energy, Δ?
A) [Cr(H2O)6]3+
B) [Cr(SCN)6]3-
C) [Cr(NH3)6]3+
D) [Cr(CN)6]3-
E) [Cr(en)3]3+ (en = ethylenediamine)
A) [Cr(H2O)6]3+
B) [Cr(SCN)6]3-
C) [Cr(NH3)6]3+
D) [Cr(CN)6]3-
E) [Cr(en)3]3+ (en = ethylenediamine)

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
42
Consider the following octahedral complex structures, each involving ethylene diamine and two different, unidentate ligands X and Y. 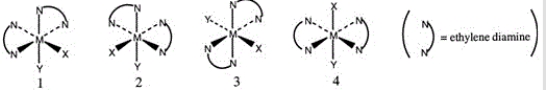 Which one of the following statements about these structures is incorrect?
Which one of the following statements about these structures is incorrect?
A) Structures 1 and 2 are optical isomers.
B) Structures 1 and 3 are optical isomers.
C) Structures 1 and 3 are different complexes.
D) Structures 1 and 4 are geometrical isomers.
E) Structures 3 and 4 are the same complex.
 Which one of the following statements about these structures is incorrect?
Which one of the following statements about these structures is incorrect?A) Structures 1 and 2 are optical isomers.
B) Structures 1 and 3 are optical isomers.
C) Structures 1 and 3 are different complexes.
D) Structures 1 and 4 are geometrical isomers.
E) Structures 3 and 4 are the same complex.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following ions could exist in either the high-spin or low-spin state in an octahedral complex?
A) Sc3+
B) Ni2+
C) Mn2+
D) Ti4+
E) Zn2+
A) Sc3+
B) Ni2+
C) Mn2+
D) Ti4+
E) Zn2+

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following ions could exist in only the high-spin state in an octahedral complex?
A) Cr2+
B) Mn4+
C) Fe3+
D) Co3+
E) Ni2+
A) Cr2+
B) Mn4+
C) Fe3+
D) Co3+
E) Ni2+

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
45
Write the formula for diamminedichloroethylenediaminecobalt(III) bromide.
A)[CoCl2(en)(NH3)2]Br
B)[CoCl2(en)(NH3)2]Br2
C)[CoCl2(en)2(NH3)2]Br
D)[CoCl2(en)2(NH3)2]Br2
E) None of these choices are correct.
A)[CoCl2(en)(NH3)2]Br
B)[CoCl2(en)(NH3)2]Br2
C)[CoCl2(en)2(NH3)2]Br
D)[CoCl2(en)2(NH3)2]Br2
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following ligands could participate in linkage isomerism?
A) NH3
B)H2O
C)NH4+
D)NO2-
E) ethylenediamine
A) NH3
B)H2O
C)NH4+
D)NO2-
E) ethylenediamine

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
47
Consider the following octahedral complex structures, each involving ethylene diamine and two different, unidentate ligands X and Y. 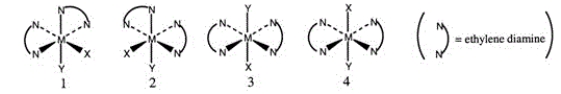 Which one, if any, of the following is a pair of optical isomers?
Which one, if any, of the following is a pair of optical isomers?
A) 1 and 2
B) 1 and 3
C) 1 and 4
D) 3 and 4
E) None of these choices are correct.
 Which one, if any, of the following is a pair of optical isomers?
Which one, if any, of the following is a pair of optical isomers?A) 1 and 2
B) 1 and 3
C) 1 and 4
D) 3 and 4
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
48
Write the formula for pentaamminechlorocobalt(III) chloride.
A) [Co(NH3)5Cl]Cl
B)[Co(NH3)5Cl]Cl2
C)[Co(NH3)5Cl]Cl3
D)[Co(NH3)5Cl]Cl4
E) None of these choices are correct.
A) [Co(NH3)5Cl]Cl
B)[Co(NH3)5Cl]Cl2
C)[Co(NH3)5Cl]Cl3
D)[Co(NH3)5Cl]Cl4
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
49
In a coordination compound involving a complex ion of square planar geometry, which of the following types of isomerism is/are never possible?
A) Geometric
B) Optical
C) Linkage
D) Coordination
E) More than one of these
A) Geometric
B) Optical
C) Linkage
D) Coordination
E) More than one of these

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
50
According to valence bond theory, what would be the set of hybrid orbitals used when a Period 4 transition metal forms a square planar complex?
A)d2sp
B)d2p2
C)dsp3
D)sp3
E)dsp2
A)d2sp
B)d2p2
C)dsp3
D)sp3
E)dsp2

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following species could exist as isomers?
A)[Co(H2O)4Cl2]+
B)[Pt(NH3)Br3]-
C)[Pt(en)Cl2]
D)[Pt(NH3)3Cl]+
E)None of these choices are correct.
A)[Co(H2O)4Cl2]+
B)[Pt(NH3)Br3]-
C)[Pt(en)Cl2]
D)[Pt(NH3)3Cl]+
E)None of these choices are correct.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
52
Write the formula for sodium tetracyanonickelate(II).
A) Na[Ni(CN)4]
B) Na[Ni(CN)4]2
C) Na2[Ni(CN)4]
D) Na4[Ni(CN)4]
E) None of these choices are correct.
A) Na[Ni(CN)4]
B) Na[Ni(CN)4]2
C) Na2[Ni(CN)4]
D) Na4[Ni(CN)4]
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
53
According to valence bond theory, what would be the set of hybrid orbitals used when a Period 4 transition metal forms a tetrahedral complex?
A)d2sp
B)dsp2
C)dsp3
D)sp3
E)d2p2
A)d2sp
B)dsp2
C)dsp3
D)sp3
E)d2p2

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
54
Give the systematic name for [CoCl3(H2O)]-.
A) cobalt(II) chloride monohydrate
B)aquatrichlorocobalt(II)
C)aquatrichlorocobaltate(II)
D)aquatrichlorocobaltite(I)
E)None of these choices are correct.
A) cobalt(II) chloride monohydrate
B)aquatrichlorocobalt(II)
C)aquatrichlorocobaltate(II)
D)aquatrichlorocobaltite(I)
E)None of these choices are correct.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
55
In the presence of a strong octahedral ligand field, the number of unpaired electrons in Co(III) will be
A) 0.
B) 2.
C) 4.
D) 6.
E) None of these choices are correct.
A) 0.
B) 2.
C) 4.
D) 6.
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
56
According to Valence Bond theory, in the square planar Ni(CN)42- complex ion, the orbital hybridization pattern is
A)sp3.
B)dsp2.
C)d2sp.
D)d2sp3.
E) None of these choices are correct.
A)sp3.
B)dsp2.
C)d2sp.
D)d2sp3.
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
57
Consider the following structures (1 and 2 are octahedral; 3 and 4 are square planar). 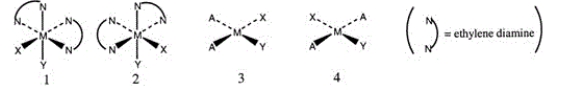 Which one of the following statements about the above structures is correct?
Which one of the following statements about the above structures is correct?
A) 1 and 2 are superimposable.
B)1 and 2 are geometric isomers.
C)3 and 4 are structural isomers.
D)3 and 4 are optical isomers.
E)3 and 4 are geometric isomers.
 Which one of the following statements about the above structures is correct?
Which one of the following statements about the above structures is correct?A) 1 and 2 are superimposable.
B)1 and 2 are geometric isomers.
C)3 and 4 are structural isomers.
D)3 and 4 are optical isomers.
E)3 and 4 are geometric isomers.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
58
In the spectrochemical series, which one of the following ligands has the strongest field?
A)H2O
B) CN-
C) NH3
D) OH-
E) Cl-
A)H2O
B) CN-
C) NH3
D) OH-
E) Cl-

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following ligands is most likely to form a low-spin octahedral complex with iron(III)?
A) Cl?
B) H2O
C) NH3
D) OH-
E) CO
A) Cl?
B) H2O
C) NH3
D) OH-
E) CO

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
60
The crystal field splitting energy, Δ,
A) is larger for tetrahedral complexes than for octahedral complexes.
B) depends on the metal but not on the ligand.
C) determines the color of a complex.
D) is larger for ionic ligands like chloride than for molecular ligands like carbon monoxide, CO.
E) determines the charge of a complex.
A) is larger for tetrahedral complexes than for octahedral complexes.
B) depends on the metal but not on the ligand.
C) determines the color of a complex.
D) is larger for ionic ligands like chloride than for molecular ligands like carbon monoxide, CO.
E) determines the charge of a complex.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
61
Of the 3d transition series of elements, scandium has the greatest atomic radius.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
62
Iron(III) forms an octahedral complex with the ligand CN-. How many unpaired electrons are in the d orbitals of iron?
A) 1
B) 3
C) 5
D) 7
E) None of these choices are correct.
A) 1
B) 3
C) 5
D) 7
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
63
The ground state electron configuration of a transition element atom cannot have more than one incomplete subshell.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
64
How many unpaired electrons will there be in a high-spin octahedral complex of Fe(II)?
A) 0
B) 2
C) 4
D) 6
E) None of these choices are correct.
A) 0
B) 2
C) 4
D) 6
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
65
Square planar complexes can exhibit both geometric and optical isomerism.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
66
Octahedral complexes can exhibit geometric, optical, and linkage isomerism.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
67
Tetrahedral complexes can exhibit both optical and linkage isomerism.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
68
The inner transition series of elements arise from the filling of f orbitals.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
69
The maximum oxidation state of an element in the first transition series never exceeds its group number.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
70
The M2+ ions of the first transition series of elements all have the general electronic configuration [Ar]4s23dx, where x is an integer from 1 to 8.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
71
All the actinide series of transition elements are radioactive.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
72
The permanganate ion (MnO4-) is a powerful reducing agent.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
73
In complexes of transition metals, the maximum coordination number of the metal is equal to its number of d electrons.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
74
All atoms of the first transition series of elements have the ground state electronic configuration [Ar]4s23dx, where x is an integer from 1 to 10.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following ligands is most likely to form a high spin octahedral complex with cobalt(II)?
A) CN-
B) en (ethylenediamine)
C) NO2-
D) CO
E) I?
A) CN-
B) en (ethylenediamine)
C) NO2-
D) CO
E) I?

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
76
If a solution absorbs green light, what is its likely color?
A) red
B) violet
C) orange
D) yellow
E) blue
A) red
B) violet
C) orange
D) yellow
E) blue

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
77
Valence Bond theory rationalizes octahedral geometry by assuming a d2sp3 hybridization pattern.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
78
The conversion of the chromate ion (CrO42-) to the dichromate ion (Cr2O72-) is a redox process.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
79
Of the 3d transition series of elements, zinc has the greatest atomic radius.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
80
The Cu2+ ion has 1 unpaired electron.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck


