Deck 20: Organic Chemistry I: Structures
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/109
Play
Full screen (f)
Deck 20: Organic Chemistry I: Structures
1
What is the maximum number of covalent bonds that a carbon atom can form?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5
4
2
Which of the following acyclic compounds is an alkane?
A) C10H22
B) C11H22
C) C12H22
D) C5H10
E) C5H8
A) C10H22
B) C11H22
C) C12H22
D) C5H10
E) C5H8
C10H22
3
Identify the hybridization of carbon atoms numbered 1-6 in the structure below: 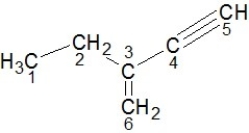
A) Carbons 1 and 2 are sp3, carbons 3 and 6 are sp2, carbons 4 and 5 are sp hybridized.
B) Carbons 1 and 3 are sp3, carbons 2 and 5 are sp2, carbons 4 and 6 are sp hybridized.
C) Carbons 2 and 3 are sp3, carbons 4 and 5 are sp2, carbons 1 and 6 are sp hybridized.
D) Carbons 4 and 5 are sp3, carbons 3 and 6 are sp2, carbons 1 and 2 are sp hybridized.
E) Carbons 3 and 6 are sp3, carbons 4 and 5 are sp2, carbons 1 and 2 are sp hybridized.

A) Carbons 1 and 2 are sp3, carbons 3 and 6 are sp2, carbons 4 and 5 are sp hybridized.
B) Carbons 1 and 3 are sp3, carbons 2 and 5 are sp2, carbons 4 and 6 are sp hybridized.
C) Carbons 2 and 3 are sp3, carbons 4 and 5 are sp2, carbons 1 and 6 are sp hybridized.
D) Carbons 4 and 5 are sp3, carbons 3 and 6 are sp2, carbons 1 and 2 are sp hybridized.
E) Carbons 3 and 6 are sp3, carbons 4 and 5 are sp2, carbons 1 and 2 are sp hybridized.
Carbons 1 and 2 are sp3, carbons 3 and 6 are sp2, carbons 4 and 5 are sp hybridized.
4
Identify the generic formula for alkenes.
A) CnH2n+4
B) CnH2n+-2
C) CnH2n+1
D) CnH2n
E) CnH2n-4
A) CnH2n+4
B) CnH2n+-2
C) CnH2n+1
D) CnH2n
E) CnH2n-4

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following compounds is a ketone?
A) CH3CH2CH2CO2H
B)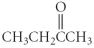
C) CH3CH2NH2
D)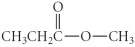
E)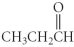
A) CH3CH2CH2CO2H
B)
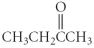
C) CH3CH2NH2
D)
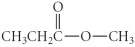
E)
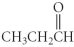

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following acyclic compounds is an alkene with one double bond in its structure?
A) C10H22
B) C11H22
C) C12H22
D) C6H10
E) C5H8
A) C10H22
B) C11H22
C) C12H22
D) C6H10
E) C5H8

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following compounds is an alcohol?
A) CH3CH2CH2C(=O)OH
B) CH3-O-CH2CH3
C) CH3CO2CH3
D) CH2OH-CH2OH
E) CH3CH2CH=O
A) CH3CH2CH2C(=O)OH
B) CH3-O-CH2CH3
C) CH3CO2CH3
D) CH2OH-CH2OH
E) CH3CH2CH=O

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following acyclic compounds is an alkyne with one triple bond in its structure?
A) C10H22
B) C11H22
C) C12H22
D) C9H18
E) C5H10
A) C10H22
B) C11H22
C) C12H22
D) C9H18
E) C5H10

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following functional groups is found in amines?
A) -C≡N
B) -NO2
C) -NH2
D) -N≡N
E) -N=NH
A) -C≡N
B) -NO2
C) -NH2
D) -N≡N
E) -N=NH

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements regarding functional groups and families is TRUE?
A) A group of organic compounds forms a family if they share the same characteristic carbon atom backbone called the functional group.
B) A group of organic compounds forms a family if they share the same characteristic atom or a group of atoms called the functional group.
C) A group of organic compounds forms a family if they differ by one CH group, called the functional group, in their structure.
D) A family of organic compounds are all structural isomers with the same molecular formula called the functional group formula.
E) A family of organic compounds is composed of all isomers that have the same molecular formula and share the same characteristic atom or a group of atoms called the functional group.
A) A group of organic compounds forms a family if they share the same characteristic carbon atom backbone called the functional group.
B) A group of organic compounds forms a family if they share the same characteristic atom or a group of atoms called the functional group.
C) A group of organic compounds forms a family if they differ by one CH group, called the functional group, in their structure.
D) A family of organic compounds are all structural isomers with the same molecular formula called the functional group formula.
E) A family of organic compounds is composed of all isomers that have the same molecular formula and share the same characteristic atom or a group of atoms called the functional group.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following compounds is a carboxylic acid?
A)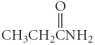
B)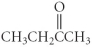
C)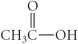
D)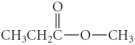
E)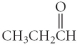
A)
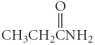
B)
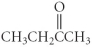
C)
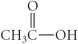
D)
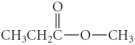
E)
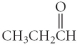

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following statements is TRUE?
A) Organic halides are polar molecules with C-X bond polarity increasing in order C-Br < C-I < C-Cl < C-F.
B) Alkenes are hydrocarbons that contain one or more C=C bonds and therefore must have some carbons sp hybridized.
C) Amines are organic compounds that contain nitrogen and have R-C(=O)-NH-R as their general formula.
D) Ethers are a family of organic compounds that contain oxygen and have the general formula R-O-R.
E) Because alcohols contain nonpolar C-H bonds, they cannot mix with water.
A) Organic halides are polar molecules with C-X bond polarity increasing in order C-Br < C-I < C-Cl < C-F.
B) Alkenes are hydrocarbons that contain one or more C=C bonds and therefore must have some carbons sp hybridized.
C) Amines are organic compounds that contain nitrogen and have R-C(=O)-NH-R as their general formula.
D) Ethers are a family of organic compounds that contain oxygen and have the general formula R-O-R.
E) Because alcohols contain nonpolar C-H bonds, they cannot mix with water.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
13
Which one of the following structures belongs to the amide family?
A)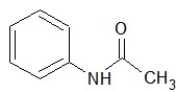
B)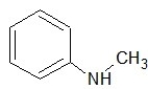
C)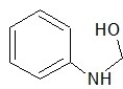
D)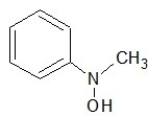
E)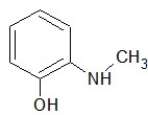
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
14
Identify the generic formula for alkanes.
A) CnH2n+2
B) CnH2n-2
C) CnH2n
D) CnH2n-4
E) CnH2n+4
A) CnH2n+2
B) CnH2n-2
C) CnH2n
D) CnH2n-4
E) CnH2n+4

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following compounds is an aldehyde?
A) CH3CH2CH=O
B) CH3CH2CH2CO2H
C) CH3CO2CH3
D) CH3-O-CH3
E) CH2OH-CH2OH
A) CH3CH2CH=O
B) CH3CH2CH2CO2H
C) CH3CO2CH3
D) CH3-O-CH3
E) CH2OH-CH2OH

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following compounds is an ester?
A)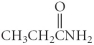
B)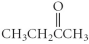
C)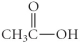
D)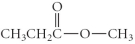
E)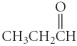
A)
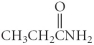
B)
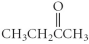
C)
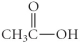
D)
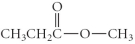
E)
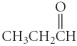

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following compounds is an amine?
A) (CH3CH2)2NH
B) CH3CH2CH2CH2CO2CH3
C) CH3CH2CH2CH2-O-CH2CH2CH3
D) CH3CH=O
E) CH3COCH3
A) (CH3CH2)2NH
B) CH3CH2CH2CH2CO2CH3
C) CH3CH2CH2CH2-O-CH2CH2CH3
D) CH3CH=O
E) CH3COCH3

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
18
Identify hybridization of carbon atoms numbered 1-6 in the structure below: 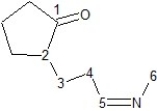
A) Carbons 3, 4, and 6 are sp3, carbons 1, 2 and 5 are sp hybridized.
B) Carbons 2, 3, and 4 are sp3, carbons 1, 5, and 6 are sp2 hybridized.
C) Carbons 2, 3, 4, and 6 are sp3, carbons 1 and 5 are sp2 hybridized.
D) Carbons 2, 3, 4, and 6 are sp3, carbons 1 and 5 are sp hybridized.
E) Carbons 3, 4, and 6 are sp3, carbons 1, 2, and 5 are sp2 hybridized.

A) Carbons 3, 4, and 6 are sp3, carbons 1, 2 and 5 are sp hybridized.
B) Carbons 2, 3, and 4 are sp3, carbons 1, 5, and 6 are sp2 hybridized.
C) Carbons 2, 3, 4, and 6 are sp3, carbons 1 and 5 are sp2 hybridized.
D) Carbons 2, 3, 4, and 6 are sp3, carbons 1 and 5 are sp hybridized.
E) Carbons 3, 4, and 6 are sp3, carbons 1, 2, and 5 are sp2 hybridized.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
19
When does conjugation occur?
A) Conjugation occurs every time an organic molecule has a heteroatom in its structure regardless of hybridization.
B) Conjugation occurs every time an organic molecule has a sequence of three or more sp2 hybridized atoms.
C) Conjugation occurs every time an organic molecule has a sequence of three or more sp3 hybridized atoms.
D) Conjugation occurs every time an organic molecule has a sequence of two or more sp2 hybridized atoms.
E) Conjugation occurs every time an organic molecule has a ring-type structure.
A) Conjugation occurs every time an organic molecule has a heteroatom in its structure regardless of hybridization.
B) Conjugation occurs every time an organic molecule has a sequence of three or more sp2 hybridized atoms.
C) Conjugation occurs every time an organic molecule has a sequence of three or more sp3 hybridized atoms.
D) Conjugation occurs every time an organic molecule has a sequence of two or more sp2 hybridized atoms.
E) Conjugation occurs every time an organic molecule has a ring-type structure.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following compounds is an ether?
A)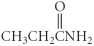
B)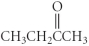
C)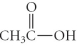
D)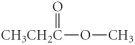
E)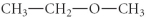
A)
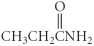
B)
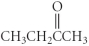
C)
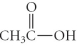
D)
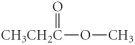
E)
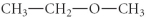

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following structures shows a primary amine?
A)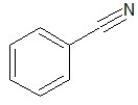
B)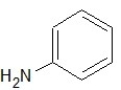
C)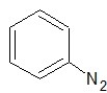
D)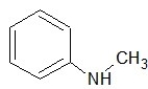
E)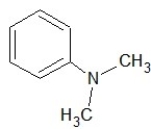
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following alcohols has the lowest solubility in water?
A) CH3CH2OH
B) CH3CH2CH2CH2OH
C) CH3CH2CH2CH2CH2OH
D) CH3CH2CH2CH2CH2CH2OH
E) CH3CH2CH2CH2CH2CH2CH2CH2OH
A) CH3CH2OH
B) CH3CH2CH2CH2OH
C) CH3CH2CH2CH2CH2OH
D) CH3CH2CH2CH2CH2CH2OH
E) CH3CH2CH2CH2CH2CH2CH2CH2OH

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following organic families contains nearly nonpolar compounds?
A) carboxylic acids
B) alcohols
C) amines
D) esters
E) ethers
A) carboxylic acids
B) alcohols
C) amines
D) esters
E) ethers

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following alcohols has the highest solubility in water?
A) CH3CH2OH
B) CH3CH2CH2CH2OH
C) CH3CH2CH2CH2CH2OH
D) CH3CH2CH2CH2CH2CH2OH
E) CH3CH2CH2CH2CH2CH2CH2CH2OH
A) CH3CH2OH
B) CH3CH2CH2CH2OH
C) CH3CH2CH2CH2CH2OH
D) CH3CH2CH2CH2CH2CH2OH
E) CH3CH2CH2CH2CH2CH2CH2CH2OH

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
25
Which one of the following molecules is the most polar?
A) butane
B) acetic acid
C) cyclohexane
D) methanol
E) cyclohexanone
A) butane
B) acetic acid
C) cyclohexane
D) methanol
E) cyclohexanone

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following condensed general formulas represents tertiary alcohols?
A) RCH2OR
B) RCH2OH
C) R3COR
D) R3COH
E) R3CCH2OH
A) RCH2OR
B) RCH2OH
C) R3COR
D) R3COH
E) R3CCH2OH

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following compounds is ethanol?
A) C2H6
B) C2H5OH
C) CH3CO2H
D) CH3CO2CH3
E) CH3-O-CH3
A) C2H6
B) C2H5OH
C) CH3CO2H
D) CH3CO2CH3
E) CH3-O-CH3

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is a secondary alcohol?
A) CH3CH2OH
B) (CH3)2(CH3CH2)COH
C) (CH3)3COH
D) (CH3)2CHCH(CH3)OH
E) CH3OCH3
A) CH3CH2OH
B) (CH3)2(CH3CH2)COH
C) (CH3)3COH
D) (CH3)2CHCH(CH3)OH
E) CH3OCH3

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following molecules is the least polar?
A) octanol
B) cyclohexane
C) ethylamine
D) dodecanol
E) cyclohexanone
A) octanol
B) cyclohexane
C) ethylamine
D) dodecanol
E) cyclohexanone

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is a primary alcohol?
A) CH3CH2OH
B) (CH3)2CHOH
C) (CH3)3COH
D) (CH3)2CHCH(CH3)OH
E) CH3OCH3
A) CH3CH2OH
B) (CH3)2CHOH
C) (CH3)3COH
D) (CH3)2CHCH(CH3)OH
E) CH3OCH3

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following condensed general formulas represents alcohols?
A) ROR
B) RCOR
C) RCHO
D) ROH
E) RCOOH
A) ROR
B) RCOR
C) RCHO
D) ROH
E) RCOOH

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following statements correctly describes organic halides?
A) Organic halides have one or more H atoms substituted by an electronegative atom from group 16 of the periodic table.
B) Organic halides have one or more C atoms substituted by an electronegative atom from group 17 of the periodic table.
C) Organic halides have one or more H atoms substituted by an electronegative atom from group 17 of the periodic table.
D) Organic halides have one or more H atoms substituted by an electropositive atom from group 17 of the periodic table.
E) Organic halides have one or more H atoms substituted by an electronegative atom from group 7 of the periodic table.
A) Organic halides have one or more H atoms substituted by an electronegative atom from group 16 of the periodic table.
B) Organic halides have one or more C atoms substituted by an electronegative atom from group 17 of the periodic table.
C) Organic halides have one or more H atoms substituted by an electronegative atom from group 17 of the periodic table.
D) Organic halides have one or more H atoms substituted by an electropositive atom from group 17 of the periodic table.
E) Organic halides have one or more H atoms substituted by an electronegative atom from group 7 of the periodic table.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following compounds contain an acyl group?
A) esters, ethers, carboxylic acids
B) esters, carboxylic acids, amines
C) esters, carboxylic acids, amides
D) esters, alcohols, amines
E) ethers, amides, esters
A) esters, ethers, carboxylic acids
B) esters, carboxylic acids, amines
C) esters, carboxylic acids, amides
D) esters, alcohols, amines
E) ethers, amides, esters

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following condensed general formulas represents ethers?
A) RCOR
B) RCHO
C) ROR
D) ROH
E) RCONH2
A) RCOR
B) RCHO
C) ROR
D) ROH
E) RCONH2

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following sets of compounds contain one ether, one alcohol, and one ketone (not necessarily in this order)?
A)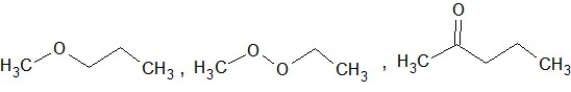
B)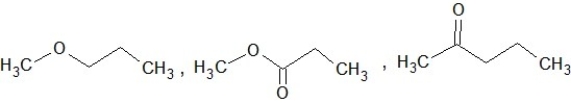
C)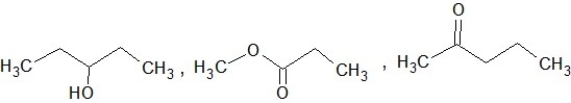
D)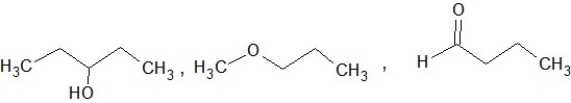
E)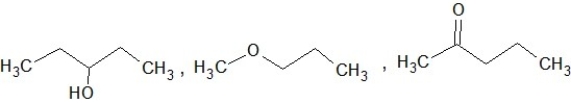
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is a tertiary alcohol?
A) CH3CH2OH
B) (CH3)2CHOH
C) (CH3)3COH
D) (CH3)2CHCH(CH3)OH
E) CH3OCH3
A) CH3CH2OH
B) (CH3)2CHOH
C) (CH3)3COH
D) (CH3)2CHCH(CH3)OH
E) CH3OCH3

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following structures shows a secondary amine?
A)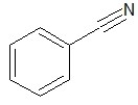
B)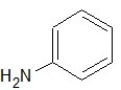
C)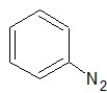
D)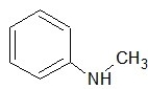
E)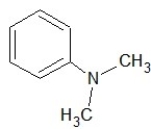
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
38
Which two functional groups combined give the functional group found in carboxylic acids?
A) carbonyl and hydroxyl
B) carbonyl and amine
C) ether and hydroxyl
D) ester and hydroxyl
E) chlorido and carbonyl
A) carbonyl and hydroxyl
B) carbonyl and amine
C) ether and hydroxyl
D) ester and hydroxyl
E) chlorido and carbonyl

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following condensed general formulas represents aldehydes?
A) ROR
B) RCOR
C) RCHO
D) ROH
E) RCOOH
A) ROR
B) RCOR
C) RCHO
D) ROH
E) RCOOH

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
40
Which compound is a saturated hydrocarbon?
A) 1-butyne
B) acetylene
C) 3-methylheptane
D) 2-methylheptene
E) ethene
A) 1-butyne
B) acetylene
C) 3-methylheptane
D) 2-methylheptene
E) ethene

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following lists the substituents in the order of priority, from highest (priority 1) to lowest (priority 4)?
A) -Cl, -CH2CH3, -CH=CH2, -CH3
B) -OH, -CH2CH3, -CH=CH2, CH3
C) -NH2, -CONH2, -COOH, CH3
D) -NH2, -COOH, -C(=O)H, -CH3
E) -Cl, -Br, -OH, -H
A) -Cl, -CH2CH3, -CH=CH2, -CH3
B) -OH, -CH2CH3, -CH=CH2, CH3
C) -NH2, -CONH2, -COOH, CH3
D) -NH2, -COOH, -C(=O)H, -CH3
E) -Cl, -Br, -OH, -H

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following pairs of organic compound families could have constitutional isomers?
A) an ether and an alcohol
B) an amide and an amine
C) an ether and a carboxylic acid
D) a carboxylic acid and an amide
E) an amine and an alcohol
A) an ether and an alcohol
B) an amide and an amine
C) an ether and a carboxylic acid
D) a carboxylic acid and an amide
E) an amine and an alcohol

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following statements is TRUE?
A) Enantiomers are molecules that are nonsuperimposable mirror images of each other and they have a chiral sp2 carbon atom with four different substituents in a tetrahedral arrangement.
B) Enantiomers are molecules that are nonsuperimposable mirror images of each other and they have a prochiral carbon atom with four different substituents in a tetrahedral arrangement.
C) Enantiomers are molecules that are superimposable mirror images of each other and they have a chiral carbon atom with four different substituents in a tetrahedral arrangement.
D) Enantiomers are molecules that are nonsuperimposable mirror images of each other and they have a chiral carbon atom with four different substituents in a planar arrangement.
E) Enantiomers are molecules that are nonsuperimposable mirror images of each other and they have a chiral carbon atom with four different substituents in a tetrahedral arrangement.
A) Enantiomers are molecules that are nonsuperimposable mirror images of each other and they have a chiral sp2 carbon atom with four different substituents in a tetrahedral arrangement.
B) Enantiomers are molecules that are nonsuperimposable mirror images of each other and they have a prochiral carbon atom with four different substituents in a tetrahedral arrangement.
C) Enantiomers are molecules that are superimposable mirror images of each other and they have a chiral carbon atom with four different substituents in a tetrahedral arrangement.
D) Enantiomers are molecules that are nonsuperimposable mirror images of each other and they have a chiral carbon atom with four different substituents in a planar arrangement.
E) Enantiomers are molecules that are nonsuperimposable mirror images of each other and they have a chiral carbon atom with four different substituents in a tetrahedral arrangement.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following pairs are constitutional isomers?
A) CH3CH=CHCH2OH and CH3CH=CHOCH3
B) ClCH2CH2CH2CH3 and CH3CH2CH2CH2Cl
C) CH3CH(OH)CH2CH3 and CH3CH2(HO)CHCH3
D) H2NCH2CH=CHCOOH and HOOCCH=CH(H2N)CH2
E) CH3OCH2CH2CH2CH3 and CH3OCH2CH2CH2OCH3
A) CH3CH=CHCH2OH and CH3CH=CHOCH3
B) ClCH2CH2CH2CH3 and CH3CH2CH2CH2Cl
C) CH3CH(OH)CH2CH3 and CH3CH2(HO)CHCH3
D) H2NCH2CH=CHCOOH and HOOCCH=CH(H2N)CH2
E) CH3OCH2CH2CH2CH3 and CH3OCH2CH2CH2OCH3

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is TRUE regarding constitutional isomers?
A) Constitutional isomers have the same molecular formula but different structures, physical, and chemical properties.
B) Constitutional isomers have the same molecular formula and physical properties but different structures and chemical properties.
C) Constitutional isomers have the same molecular formula, physical, and chemical properties but different structures.
D) Constitutional isomers have the same chemical structure and molecular formula but different physical and chemical properties.
E) Constitutional isomers have the same chemical structure but different formulas, physical, and chemical properties.
A) Constitutional isomers have the same molecular formula but different structures, physical, and chemical properties.
B) Constitutional isomers have the same molecular formula and physical properties but different structures and chemical properties.
C) Constitutional isomers have the same molecular formula, physical, and chemical properties but different structures.
D) Constitutional isomers have the same chemical structure and molecular formula but different physical and chemical properties.
E) Constitutional isomers have the same chemical structure but different formulas, physical, and chemical properties.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following structures shows an E alkene isomer?
A)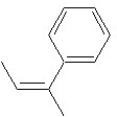
B)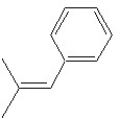
C)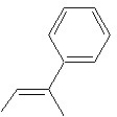
D)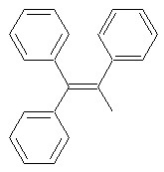
E)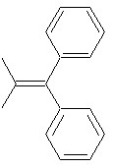
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following structures shows a Z alkene isomer?
A)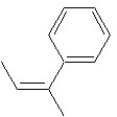
B)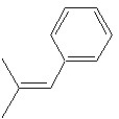
C)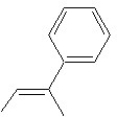
D)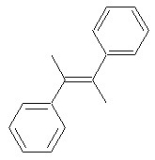
E)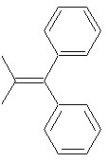
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is a constitutional isomer of the following organic molecule? 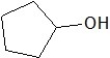
A)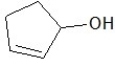
B)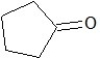
C)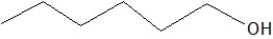
D)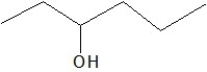
E)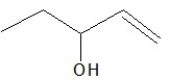

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following compounds exhibit optical isomerism?
A) sec-butylbenzene
B) CH2Cl2
C) CF2BrI
D) CHBr2I
E) tert-butylbenzene
A) sec-butylbenzene
B) CH2Cl2
C) CF2BrI
D) CHBr2I
E) tert-butylbenzene

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
50
Find the correct Newman projection formula showing a staggered conformer of n-hexane.
A)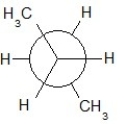
B)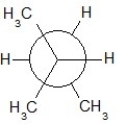
C)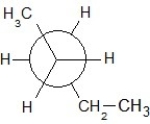
D)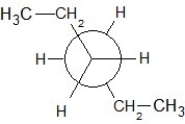
E)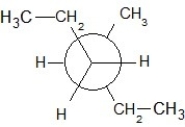
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
51
What is angle strain in cycloalkanes?
A) Angle strain is the angle between carbon bonds on the outer side of a cycloalkane ring.
B) Angle strain is the difference between an ideal bond angle for sp3 hybridized carbon atoms and the actual bond angle in the ring.
C) Angle strain is the difference between and ideal bond angle for sp hybridized carbon atoms and the actual bond angle in the ring.
D) Angle strain is the angle between C-C bonds in the cycloalkane ring.
E) Angle strain is the difference between the C-C bond angles found in a flat ring structure and in an actual ring structure.
A) Angle strain is the angle between carbon bonds on the outer side of a cycloalkane ring.
B) Angle strain is the difference between an ideal bond angle for sp3 hybridized carbon atoms and the actual bond angle in the ring.
C) Angle strain is the difference between and ideal bond angle for sp hybridized carbon atoms and the actual bond angle in the ring.
D) Angle strain is the angle between C-C bonds in the cycloalkane ring.
E) Angle strain is the difference between the C-C bond angles found in a flat ring structure and in an actual ring structure.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following structures are constitutional isomers of a compound with molecular formula C4H9N?
A)
B)
C)
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following Newman projections shows a staggered conformer of 2-methyl-butane?
A)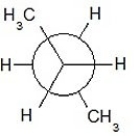
B)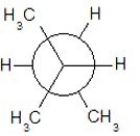
C)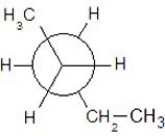
D)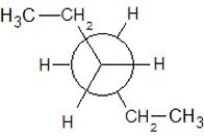
E)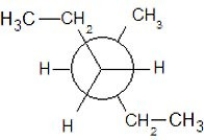
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following compounds exhibit cis-trans isomerism?
A) CH2=CH-CH3
B) CCl2=CBr2
C) CH3-CH=CH-CH3
D) CCl2=CHBr
E) (CH3)2C=CH(CH3)
A) CH2=CH-CH3
B) CCl2=CBr2
C) CH3-CH=CH-CH3
D) CCl2=CHBr
E) (CH3)2C=CH(CH3)

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following is TRUE regarding stereoisomers?
A) Stereoisomers differ in the way atoms are connected but have identical spatial orientation of atoms. They can be conformational or configurational.
B) Stereoisomers differ in the way atoms are connected but have identical spatial orientation of atoms. They can be constitutional or configurational.
C) Stereoisomers have the same atom connectivity but have different spatial orientation of atoms. They can be constitutional or enantiomeric.
D) Stereoisomers have the same atom connectivity but have different spatial orientation of atoms. They can be conformational or configurational.
E) Stereoisomers have the same atom connectivity but have different functional groups on the same positions of the carbon backbone. They can be conformational or configurational.
A) Stereoisomers differ in the way atoms are connected but have identical spatial orientation of atoms. They can be conformational or configurational.
B) Stereoisomers differ in the way atoms are connected but have identical spatial orientation of atoms. They can be constitutional or configurational.
C) Stereoisomers have the same atom connectivity but have different spatial orientation of atoms. They can be constitutional or enantiomeric.
D) Stereoisomers have the same atom connectivity but have different spatial orientation of atoms. They can be conformational or configurational.
E) Stereoisomers have the same atom connectivity but have different functional groups on the same positions of the carbon backbone. They can be conformational or configurational.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following compounds exhibit cis-trans isomerism?
A) CH2=CH2
B) CH2=CCl2
C) CBr2=CHBr
D) CHCl=CHCl
E) (CH3)2C=CH-CH2CH3
A) CH2=CH2
B) CH2=CCl2
C) CBr2=CHBr
D) CHCl=CHCl
E) (CH3)2C=CH-CH2CH3

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
57
Find the correct statement about cis-trans isomers.
A) Cis-trans isomers are diastereomers and occur because the rotation around a C≡C bond is very restricted.
B) Cis-trans isomers are enantiomers and occur because the rotation around a C=C bond is very restricted.
C) Cis-trans isomers are diastereomers and occur because the rotation around a C=C bond is very restricted.
D) Cis-trans isomers are superimposable constitutional isomers of substituted alkenes formed around a C=C bond.
E) Cis-trans isomers are chiral molecules and occur because the rotation around a C=C bond is very restricted.
A) Cis-trans isomers are diastereomers and occur because the rotation around a C≡C bond is very restricted.
B) Cis-trans isomers are enantiomers and occur because the rotation around a C=C bond is very restricted.
C) Cis-trans isomers are diastereomers and occur because the rotation around a C=C bond is very restricted.
D) Cis-trans isomers are superimposable constitutional isomers of substituted alkenes formed around a C=C bond.
E) Cis-trans isomers are chiral molecules and occur because the rotation around a C=C bond is very restricted.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following Newman projections shows an antistaggered conformation of n-pentane?
A)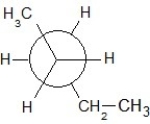
B)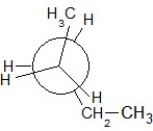
C)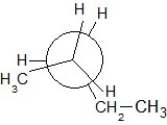
D)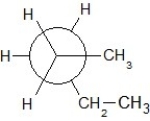
E)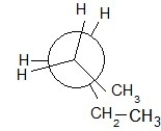
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following lists the substituents in the order of priority, from highest (priority 1) to lowest (priority 4)?
A) -CH3, -CH=CH2, -CH2CH3, -Cl
B) -CH3, -CH2CH3, -CH=CH2, -Cl
C) -Cl, -CH2CH3, -CH3, -CH=CH2
D) -Cl, -CH2CH3, -CH=CH2, -CH3
E) -Cl, -CH=CH2, -CH2CH3, -CH3
A) -CH3, -CH=CH2, -CH2CH3, -Cl
B) -CH3, -CH2CH3, -CH=CH2, -Cl
C) -Cl, -CH2CH3, -CH3, -CH=CH2
D) -Cl, -CH2CH3, -CH=CH2, -CH3
E) -Cl, -CH=CH2, -CH2CH3, -CH3

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following lists the substituents in the order of priority, from highest (priority 1) to lowest (priority 4)?
A) -NH2, -C(=O)H, -COOH, -CH3
B) -CH3, -COOH, -C(=O)H, -NH2
C) -NH2, -CH3, -C(=O)H, -COOH
D) -NH2, -COOH, -C(=O)H, -CH3
E) -NH2, -CH3, -COOH, -C(=O)H
A) -NH2, -C(=O)H, -COOH, -CH3
B) -CH3, -COOH, -C(=O)H, -NH2
C) -NH2, -CH3, -C(=O)H, -COOH
D) -NH2, -COOH, -C(=O)H, -CH3
E) -NH2, -CH3, -COOH, -C(=O)H

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
61
The general formula for alkanes is CnH2n+2. What is the value of n in 3,7-dimethyldecane? How many chiral centres does this molecule have?
A) n = 10; there are two chiral centres
B) n = 10; there is one chiral centre
C) n = 12; there are two chiral centres
D) n = 12; there are three chiral centres
E) n = 12; there is one chiral centre
A) n = 10; there are two chiral centres
B) n = 10; there is one chiral centre
C) n = 12; there are two chiral centres
D) n = 12; there are three chiral centres
E) n = 12; there is one chiral centre

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
62
Determine the absolute configuration of the molecule shown below: 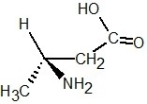
A) S configuration
B) R configuration
C) E configuration
D) Z configuration
E) l configuration

A) S configuration
B) R configuration
C) E configuration
D) Z configuration
E) l configuration

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
63
How many of the carbons in the following compound are chiral centre(s)? 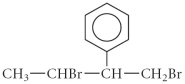
A) 0
B) 1
C) 2
D) 3
E) 4

A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
64
Determine the absolute configuration of the molecule shown below: 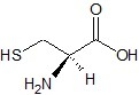
A) S configuration
B) R configuration
C) E configuration
D) Z configuration
E) l configuration

A) S configuration
B) R configuration
C) E configuration
D) Z configuration
E) l configuration

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following statements is TRUE?
A) Optical activity describes the property of a chiral compound to rotate the plane of polarized light.
B) Two enantiomers differ significantly in physical properties. This difference is the basis for the separation of the two enantiomers from a racemic mixture.
C) A racemic mixture is an optically active mixture of two enantiomers.
D) It is possible to determine if an enantiomer is the levorotatory or dextrorotatory isomer by analyzing its infrared spectrum.
E) If an enantiomer has an S absolute configuration then it must be the levorotatory isomer.
A) Optical activity describes the property of a chiral compound to rotate the plane of polarized light.
B) Two enantiomers differ significantly in physical properties. This difference is the basis for the separation of the two enantiomers from a racemic mixture.
C) A racemic mixture is an optically active mixture of two enantiomers.
D) It is possible to determine if an enantiomer is the levorotatory or dextrorotatory isomer by analyzing its infrared spectrum.
E) If an enantiomer has an S absolute configuration then it must be the levorotatory isomer.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
66
What is the index of hydrogen deficiency in C10H12N2O3?
A) 5
B) 4
C) 3
D) 2
E) 6
A) 5
B) 4
C) 3
D) 2
E) 6

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following structures is S-2-chlorobutane?
A)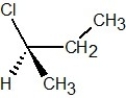
B)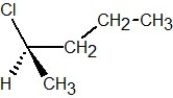
C)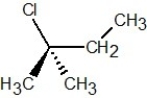
D)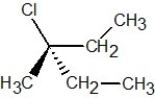
E)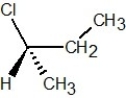
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
68
The general formula for alkanes is CnH2n+2. What is the value of n in 3-ethyl-4,7-dimethylnonane? How many chiral centres does this molecule have?
A) n = 9; there are two chiral centres
B) n = 9; there are three chiral centres
C) n = 13; there are two chiral centres
D) n = 13; there are three chiral centres
E) n = 13; there is one chiral centre
A) n = 9; there are two chiral centres
B) n = 9; there are three chiral centres
C) n = 13; there are two chiral centres
D) n = 13; there are three chiral centres
E) n = 13; there is one chiral centre

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
69
In which of the following structures have the group priorities been assigned correctly?
A)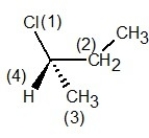
B)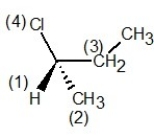
C)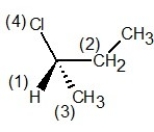
D)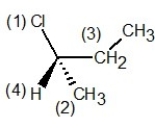
E)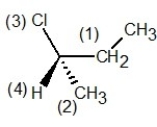
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
70
How many of the carbons in the following compound are chiral centre(s)? 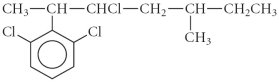
A) 0
B) 1
C) 2
D) 3
E) 4 or more
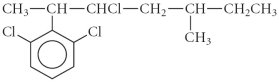
A) 0
B) 1
C) 2
D) 3
E) 4 or more

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following structures are enantiomers?
A)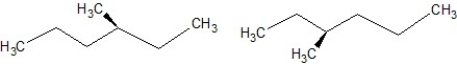
B)
C)
D)
E)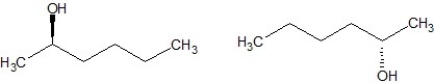
A)

B)
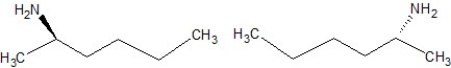
C)

D)
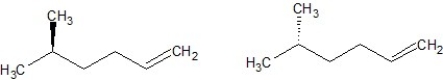
E)


Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
72
What is the index of hydrogen deficiency in C20H36?
A) 5
B) 4
C) 3
D) 2
E) 1
A) 5
B) 4
C) 3
D) 2
E) 1

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
73
Determine the absolute configuration of the molecule shown below: 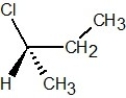
A) R configuration
B) S configuration
C) E configuration
D) Z configuration
E) d configuration

A) R configuration
B) S configuration
C) E configuration
D) Z configuration
E) d configuration

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following have correct assignments of both group priorities and absolute configurations?
A)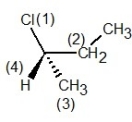 , R configuration
, R configuration
B)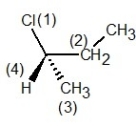 , S configuration
, S configuration
C)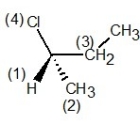 , S configuration
, S configuration
D)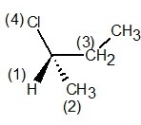 , l configuration
, l configuration
E)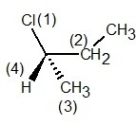 , d configuration
, d configuration
A)
 , R configuration
, R configurationB)
 , S configuration
, S configurationC)
 , S configuration
, S configurationD)
 , l configuration
, l configurationE)
 , d configuration
, d configuration
Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following compounds is chiral?
A) 2-methylpentane
B) 3-ethylhexane
C) 3-methylheptane
D) 4-ethylheptane
E) 3-methylpentane
A) 2-methylpentane
B) 3-ethylhexane
C) 3-methylheptane
D) 4-ethylheptane
E) 3-methylpentane

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
76
What is the index of hydrogen deficiency in C9H16Br2?
A) 5
B) 4
C) 3
D) 2
E) 1
A) 5
B) 4
C) 3
D) 2
E) 1

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following compounds exhibits optical isomerism?
A) CH3-CH2-CH3
B) CH3-CH2-CHBr-CH2-CH3
C) CH3-CHCl-CH3
D) CH3-CH2-CHBr-CH3
E) CH3-CH2-CBr2-CH3
A) CH3-CH2-CH3
B) CH3-CH2-CHBr-CH2-CH3
C) CH3-CHCl-CH3
D) CH3-CH2-CHBr-CH3
E) CH3-CH2-CBr2-CH3

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following structures is R-but-3-en-2-ol?
A)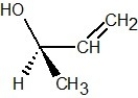
B)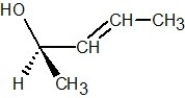
C)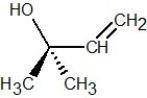
D)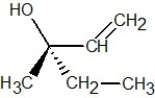
E)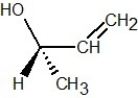
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
79
Determine the absolute configuration of the molecule shown below: 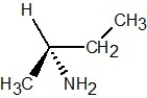
A) S configuration
B) R configuration
C) E configuration
D) Z configuration
E) d configuration

A) S configuration
B) R configuration
C) E configuration
D) Z configuration
E) d configuration

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
80
The general formula for alkanes is CnH2n+2. What is the value of n in 4-methylethylheptane? Is this molecule chiral?
A) n = 9; yes, it is chiral
B) n = 9; no, it is not chiral
C) n = 10; yes, it is chiral
D) n = 10; no, it is not chiral
E) n = 7; yes, it is chiral
A) n = 9; yes, it is chiral
B) n = 9; no, it is not chiral
C) n = 10; yes, it is chiral
D) n = 10; no, it is not chiral
E) n = 7; yes, it is chiral

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck


