Deck 20: Principles of Chemical Reactivity: Electron Transfer Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/83
Play
Full screen (f)
Deck 20: Principles of Chemical Reactivity: Electron Transfer Reactions
1
When the given oxidation-reduction reaction in an acidic solution is balanced, what is the lowest whole-number coefficient for H+, and on which side of the balanced equation should it appear? MnO4-(aq) + Br-(aq) → Mn2+(aq) + Br2(l)
A) 1; reactant side
B) 2; product side
C) 8; reactant side
D) 16; reactant side
E) 4; product side
A) 1; reactant side
B) 2; product side
C) 8; reactant side
D) 16; reactant side
E) 4; product side
16; reactant side
2
Balance the following half-reaction occurring in a basic solution. MnO2(s) → Mn(OH)2(s)
A) MnO2(s) + 2 H2O(l) + 2e−→ Mn(OH)2(s)+ 2 OH−(aq)
B) MnO2(s) + 2 H2O(l) + 4e−→ Mn(OH)2(s)+ (OH)2−(aq)
C) MnO2(s) + H22+(aq) + 2e−→ Mn(OH)2(s)
D) MnO2(s) + H2(g) → Mn(OH)2(s) + 2 e−
E) MnO2(s) + 2 H2O(l) → Mn(OH)2(s)+ 2 OH−(aq)
A) MnO2(s) + 2 H2O(l) + 2e−→ Mn(OH)2(s)+ 2 OH−(aq)
B) MnO2(s) + 2 H2O(l) + 4e−→ Mn(OH)2(s)+ (OH)2−(aq)
C) MnO2(s) + H22+(aq) + 2e−→ Mn(OH)2(s)
D) MnO2(s) + H2(g) → Mn(OH)2(s) + 2 e−
E) MnO2(s) + 2 H2O(l) → Mn(OH)2(s)+ 2 OH−(aq)
MnO2(s) + 2 H2O(l) + 2e−→ Mn(OH)2(s)+ 2 OH−(aq)
3
Write the balanced reduction half-reaction for the following overall reaction: 2 Fe(s) + 3 Cl2(aq) → 2 Fe3+(aq) + 6 Cl-(aq)
A) Cl2(aq) + 2 e- → 2 Cl-(aq)
B) Fe(s) + 3 e- → Fe3+(aq)
C) Fe(s) + Cl2(aq) → FeCl3(aq)
D) Cl2(aq) → 2 Cl-(aq) + 2 e-
E) 3 Cl2(aq) + 2 e- → 2 Cl-(aq)
A) Cl2(aq) + 2 e- → 2 Cl-(aq)
B) Fe(s) + 3 e- → Fe3+(aq)
C) Fe(s) + Cl2(aq) → FeCl3(aq)
D) Cl2(aq) → 2 Cl-(aq) + 2 e-
E) 3 Cl2(aq) + 2 e- → 2 Cl-(aq)
Cl2(aq) + 2 e- → 2 Cl-(aq)
4
When the following oxidation-reduction reaction in acidic solution is balanced, what is the lowest whole-number coefficient for Na+(aq) ion? Na(s) + Ca2+(aq) → Na+(aq) + Ca(s)
A) 5
B) 4
C) 1
D) 3
E) 2
A) 5
B) 4
C) 1
D) 3
E) 2

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
5
Balance the following oxidation-reduction reaction occurring in an acidic solution. MnO4-(aq) + Cr2+(aq) → Mn2+(aq) + Cr3+(aq)
A) MnO4-(aq) + 8 H+(aq) + 5 Cr2+(aq) → Mn2+(aq) + 4 H2O(l) + 5 Cr3+(aq)
B) MnO4-(aq) + 8 H+(aq) + Cr2+(aq) → Mn2+(aq) + 4 H2O(l) + Cr3+(aq)
C) MnO4-(aq) + 4 H2(g) + 5 Cr2+(aq)→ Mn2+(aq) + 4 H2O(l) + 5 Cr3+(aq)
D) MnO4-(aq) + 8 H+(aq) + 2 Cr2+(aq) → Mn2+(aq) + 4 H2O(l) + 2 Cr3+(aq)
E) MnO4-(aq) + Cr2+(aq) → Mn2+(aq) + 2 O2(g) + Cr3+(aq)
A) MnO4-(aq) + 8 H+(aq) + 5 Cr2+(aq) → Mn2+(aq) + 4 H2O(l) + 5 Cr3+(aq)
B) MnO4-(aq) + 8 H+(aq) + Cr2+(aq) → Mn2+(aq) + 4 H2O(l) + Cr3+(aq)
C) MnO4-(aq) + 4 H2(g) + 5 Cr2+(aq)→ Mn2+(aq) + 4 H2O(l) + 5 Cr3+(aq)
D) MnO4-(aq) + 8 H+(aq) + 2 Cr2+(aq) → Mn2+(aq) + 4 H2O(l) + 2 Cr3+(aq)
E) MnO4-(aq) + Cr2+(aq) → Mn2+(aq) + 2 O2(g) + Cr3+(aq)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following reactions will require the use of an inert electrode when used in a voltaic cell?
A) Co(s) + 2 Ag+(aq) → 2 Ag(s) + Co2+(aq)
B) 3 Ni(s) + 2 Au3+(aq) → 3 Ni2+ + 2 Au(s)
C) Cu(s) + 2 Ag+(aq) → Cu2+(aq) + 2 Ag(s)
D) Zn(s) + 2 MnO2(s) + 2 NH4+(aq) → Zn2+(aq) + Mn2O3(s) + 2 NH3(aq) + H2O(l)
E) 3 Zn2+(aq) + 2 Al(s) → 3 Zn(s) + 2 Al3+(aq)
A) Co(s) + 2 Ag+(aq) → 2 Ag(s) + Co2+(aq)
B) 3 Ni(s) + 2 Au3+(aq) → 3 Ni2+ + 2 Au(s)
C) Cu(s) + 2 Ag+(aq) → Cu2+(aq) + 2 Ag(s)
D) Zn(s) + 2 MnO2(s) + 2 NH4+(aq) → Zn2+(aq) + Mn2O3(s) + 2 NH3(aq) + H2O(l)
E) 3 Zn2+(aq) + 2 Al(s) → 3 Zn(s) + 2 Al3+(aq)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is the cell notation for a voltaic cell based on the following reaction? Cu2+(aq) + Fe(s) → Cu(s) + Fe2+(aq)
A) Cu(s) | Cu2+(aq) || Fe2+(aq) | Fe(s)
B) Fe(s) || Fe2+(aq), Cu2+(aq) | Cu(s)
C) Cu(s) || Cu2+(aq), Fe2+(aq) || Fe(s)
D) Cu(s) | Fe2+(aq) || Cu2+(aq) | Fe(s)
E) Fe(s) | Fe2+(aq) || Cu2+(aq) | Cu(s)
A) Cu(s) | Cu2+(aq) || Fe2+(aq) | Fe(s)
B) Fe(s) || Fe2+(aq), Cu2+(aq) | Cu(s)
C) Cu(s) || Cu2+(aq), Fe2+(aq) || Fe(s)
D) Cu(s) | Fe2+(aq) || Cu2+(aq) | Fe(s)
E) Fe(s) | Fe2+(aq) || Cu2+(aq) | Cu(s)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
8
Write a balanced half-reaction for the reduction of CrO42-(aq) to Cr(OH)3(s) in a basic solution.
A) CrO42-(aq) + 3 OH-(aq) + 3 e- ? Cr(OH)3(s) + 2 O2(g)
B) CrO42-(aq) + 3 H+(aq) + 3 e- ? Cr(OH)3(s)
C) CrO42-(aq) + 3 H+(aq) ? Cr(OH)3(s) + 2 e-
D) CrO42-(aq) + 4 H2O( ) + 3 e- ? Cr(OH)3(s) + 5 OH-(aq)
E) CrO42-(aq) + 3 OH-(aq) ? Cr(OH)3(s) + 2 O2(g)
A) CrO42-(aq) + 3 OH-(aq) + 3 e- ? Cr(OH)3(s) + 2 O2(g)
B) CrO42-(aq) + 3 H+(aq) + 3 e- ? Cr(OH)3(s)
C) CrO42-(aq) + 3 H+(aq) ? Cr(OH)3(s) + 2 e-
D) CrO42-(aq) + 4 H2O( ) + 3 e- ? Cr(OH)3(s) + 5 OH-(aq)
E) CrO42-(aq) + 3 OH-(aq) ? Cr(OH)3(s) + 2 O2(g)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements concerning voltaic cells is not true?
A) A salt bridge allows cations and anions to move between half-cells.
B) Electrons flow from a cathode to an anode in the external circuit.
C) Oxidation occurs at an anode.
D) A voltaic cell can be used as a source of energy.
E) A voltaic cell consists of two half-cells.
A) A salt bridge allows cations and anions to move between half-cells.
B) Electrons flow from a cathode to an anode in the external circuit.
C) Oxidation occurs at an anode.
D) A voltaic cell can be used as a source of energy.
E) A voltaic cell consists of two half-cells.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
10
Write a balanced chemical equation for the following reaction in a basic solution.
ClO-(aq) + Cr(OH)3(s) ? Cl-(aq) + CrO42-(aq)
A) 3 ClO-(aq) + 2 Cr(OH)3(s) + 4 OH-(aq) ? 3 Cl-(aq) + 2 CrO42-(aq) + 5 H2O( )
B) ClO-(aq) + Cr(OH)3(s) + 3 OH-(aq) ? Cl-(aq) + CrO42-(aq) + 3 H2O( )
C) 2 ClO-(aq) + 3 Cr(OH)3(s) + 3 OH-(aq) ? 2 Cl-(aq) + 3 CrO42-(aq) + 6 H2O( )
D) 4 ClO-(aq) + Cr(OH)3(s) + 4 OH-(aq) ? Cl-(aq) + CrO42-(aq) + 6 H2O( )
E) ClO-(aq) + Cr(OH)3(s) ? Cl-(aq) + CrO42-(aq) + 3 H+(aq)
ClO-(aq) + Cr(OH)3(s) ? Cl-(aq) + CrO42-(aq)
A) 3 ClO-(aq) + 2 Cr(OH)3(s) + 4 OH-(aq) ? 3 Cl-(aq) + 2 CrO42-(aq) + 5 H2O( )
B) ClO-(aq) + Cr(OH)3(s) + 3 OH-(aq) ? Cl-(aq) + CrO42-(aq) + 3 H2O( )
C) 2 ClO-(aq) + 3 Cr(OH)3(s) + 3 OH-(aq) ? 2 Cl-(aq) + 3 CrO42-(aq) + 6 H2O( )
D) 4 ClO-(aq) + Cr(OH)3(s) + 4 OH-(aq) ? Cl-(aq) + CrO42-(aq) + 6 H2O( )
E) ClO-(aq) + Cr(OH)3(s) ? Cl-(aq) + CrO42-(aq) + 3 H+(aq)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
11
Write the balanced oxidation half-reaction for the following overall reaction: 2 H+(aq) + Ca(s) → Ca2+(aq) + H2(g)
A) Ca(s) → 2e- + Ca2+ (aq)
B) 2 H+(aq) → H2(g) + 2 e-
C) H2(g) → 2 H+(aq) + 2 e-
D) Ca(s) + 2 e- → Ca2+(aq)
E) 2Ca(s) → Ca2+(aq) + 2 e-
A) Ca(s) → 2e- + Ca2+ (aq)
B) 2 H+(aq) → H2(g) + 2 e-
C) H2(g) → 2 H+(aq) + 2 e-
D) Ca(s) + 2 e- → Ca2+(aq)
E) 2Ca(s) → Ca2+(aq) + 2 e-

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following statements is true for the following reaction, assuming the given reaction proceeds in the forward direction? Fe3+(aq) + Co(s) → Fe2+(aq) + Co2+(aq)
A) Fe3+(aq) is oxidized and Co(s) is reduced.
B) Fe3+(aq) is oxidized and Co2+(aq) is reduced.
C) Co(s) is oxidized and Fe3+(aq) is reduced.
D) Co(s) is oxidized and Co2+(aq) is reduced.
E) Fe2+(aq) is oxidized and Co(s) is reduced.
A) Fe3+(aq) is oxidized and Co(s) is reduced.
B) Fe3+(aq) is oxidized and Co2+(aq) is reduced.
C) Co(s) is oxidized and Fe3+(aq) is reduced.
D) Co(s) is oxidized and Co2+(aq) is reduced.
E) Fe2+(aq) is oxidized and Co(s) is reduced.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
13
Write a balanced chemical equation for the oxidation of solid cadmium by concentrated nitric acid, producing nitrogen dioxide gas and Cd2+(aq) ion.
A) HNO3(aq) + Cd(s) ? Cd2+(aq) + NO2(g) + OH-(aq)
B) 2 HNO3(aq) + Cd(s) ? Cd2+(aq) + 2 NO2(g) + 2 OH-(aq)
C) HNO3(aq) + Cd(s) + H+(aq) ? Cd2+(aq) + NO2(g) + H2O( )
D) 4 HNO3(aq) + Cd(s) ? Cd2+(aq) + 2 NO2(g) + 2 H2O( ) + 2 NO3-(aq)
E) HNO3(aq) + Cd(s) ? Cd2+(aq) + NO2(g)
A) HNO3(aq) + Cd(s) ? Cd2+(aq) + NO2(g) + OH-(aq)
B) 2 HNO3(aq) + Cd(s) ? Cd2+(aq) + 2 NO2(g) + 2 OH-(aq)
C) HNO3(aq) + Cd(s) + H+(aq) ? Cd2+(aq) + NO2(g) + H2O( )
D) 4 HNO3(aq) + Cd(s) ? Cd2+(aq) + 2 NO2(g) + 2 H2O( ) + 2 NO3-(aq)
E) HNO3(aq) + Cd(s) ? Cd2+(aq) + NO2(g)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
14
How many electrons are transferred in the given reaction? Ni + 2 HCl → Ni Cl2 + H2
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following statements is true for the following reaction, assuming the given reaction proceeds in the forward direction? 3 Sn4+(aq) + 2 Cr(s) → 3 Sn2+(aq) + 2 Cr3+(aq)
A) Sn4+(aq) is the reducing agent and Cr(s) is the oxidizing agent.
B) Cr(s) is the reducing agent and Sn2+(aq) is the oxidizing agent.
C) Sn4+(aq) is the reducing agent and Sn2+(aq) is the oxidizing agent.
D) Cr(s) is the reducing agent and Cr3+(aq) is the oxidizing agent.
E) Cr(s) is the reducing agent and Sn4+(aq) is the oxidizing agent.
A) Sn4+(aq) is the reducing agent and Cr(s) is the oxidizing agent.
B) Cr(s) is the reducing agent and Sn2+(aq) is the oxidizing agent.
C) Sn4+(aq) is the reducing agent and Sn2+(aq) is the oxidizing agent.
D) Cr(s) is the reducing agent and Cr3+(aq) is the oxidizing agent.
E) Cr(s) is the reducing agent and Sn4+(aq) is the oxidizing agent.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
16
Write a balanced half-reaction for the reduction of hydrogen peroxide to water in an acidic solution.
A) 2 H2O2( ) ? 2 H2O( ) + O2(g)
B) 2 H2O2( ) + 2e- ? 2 H2O( ) + O2(g)
C) H2O2( ) + 2 H+(aq) + 2 e- ? 2 H2O( )
D) H2O2( ) + 4 H+(aq) + 2 e- ? 2 H2O( ) + H2(g)
E) H2O2( ) + 2 H+(aq) + 4 e- ? 2 H2(g) + O2(g)
A) 2 H2O2( ) ? 2 H2O( ) + O2(g)
B) 2 H2O2( ) + 2e- ? 2 H2O( ) + O2(g)
C) H2O2( ) + 2 H+(aq) + 2 e- ? 2 H2O( )
D) H2O2( ) + 4 H+(aq) + 2 e- ? 2 H2O( ) + H2(g)
E) H2O2( ) + 2 H+(aq) + 4 e- ? 2 H2(g) + O2(g)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
17
In the context of the diagram given below, which of the following statements is true concerning half-cell II? ![<strong>In the context of the diagram given below, which of the following statements is true concerning half-cell II? </strong> A) [Cu<sup>2+</sup>] decreases with time, and [K+] increases with time. B) [Cu<sup>2+</sup>] increases with time, and [K+] increases with time. C) [Cu<sup>2+</sup>] decreases with time, and [K+] decreases with time. D) [Cu<sup>2+</sup>] decreases with time, and [SO<sub>4</sub>2-] decreases with time. E) [Cu<sup>2+</sup>] increases with time, and [SO<sub>4</sub>2-] increases with time.](https://storage.examlex.com/TB7130/11ead6f3_2444_8af7_9e2d_2720843cdfc8_TB7130_00.jpg)
A) [Cu2+] decreases with time, and [K+] increases with time.
B) [Cu2+] increases with time, and [K+] increases with time.
C) [Cu2+] decreases with time, and [K+] decreases with time.
D) [Cu2+] decreases with time, and [SO42-] decreases with time.
E) [Cu2+] increases with time, and [SO42-] increases with time.
![<strong>In the context of the diagram given below, which of the following statements is true concerning half-cell II? </strong> A) [Cu<sup>2+</sup>] decreases with time, and [K+] increases with time. B) [Cu<sup>2+</sup>] increases with time, and [K+] increases with time. C) [Cu<sup>2+</sup>] decreases with time, and [K+] decreases with time. D) [Cu<sup>2+</sup>] decreases with time, and [SO<sub>4</sub>2-] decreases with time. E) [Cu<sup>2+</sup>] increases with time, and [SO<sub>4</sub>2-] increases with time.](https://storage.examlex.com/TB7130/11ead6f3_2444_8af7_9e2d_2720843cdfc8_TB7130_00.jpg)
A) [Cu2+] decreases with time, and [K+] increases with time.
B) [Cu2+] increases with time, and [K+] increases with time.
C) [Cu2+] decreases with time, and [K+] decreases with time.
D) [Cu2+] decreases with time, and [SO42-] decreases with time.
E) [Cu2+] increases with time, and [SO42-] increases with time.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
18
Balance the following half-reaction occurring in an acidic solution. NO3-(aq) → NO(aq)
A) NO3-(aq) + 4 H+(aq) + 3 e− → NO(g) + 2 H2O(l)
B) NO3-(aq) + 2 H2O(l) + 3 e− → NO(g) + 4 H+(aq)
C) NO3-(aq) + 4 H+(aq) → NO(g) + 2 H2O(l) + 3 e−
D) NO3-(aq) + 3 e− → NO(g) + 4 H+(aq) + 2 H2O(l)
E) NO3-(aq) + 4 H+(aq)→ NO(g) + 2 H2O(l)
A) NO3-(aq) + 4 H+(aq) + 3 e− → NO(g) + 2 H2O(l)
B) NO3-(aq) + 2 H2O(l) + 3 e− → NO(g) + 4 H+(aq)
C) NO3-(aq) + 4 H+(aq) → NO(g) + 2 H2O(l) + 3 e−
D) NO3-(aq) + 3 e− → NO(g) + 4 H+(aq) + 2 H2O(l)
E) NO3-(aq) + 4 H+(aq)→ NO(g) + 2 H2O(l)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
19
Write a balanced net ionic equation for the reaction below in an acidic solution.
Cr2O72-(aq) + Ni(s) ? Cr3+(aq) + Ni2+(aq)
A) Cr2O72-(aq) + 3 Ni(s) + 14 H+(aq) ? 2 Cr3+(aq) + 3 Ni2+(aq) + 7 H2O( )
B) Cr2O72-(aq) + Ni(s) + 14 H+(aq) ? 2 Cr3+(aq) + Ni2+(aq) + 7 H2O( ).
C) Cr2O72-(aq) + 3 Ni(s) ? 2 Cr3+(aq) + 3 Ni2+(aq) + O2-(aq)
D) Cr2O72-(aq) + Ni(s) + 14 H+(aq) ? 2 Cr3+(aq) + Ni2+(aq) + 7 H2O( )
E) Cr2O72-(aq) + 3 Ni(s) + 7 H+(aq) ? 2 Cr3+(aq) + 3 Ni2+(aq) + 7 OH-(aq)
Cr2O72-(aq) + Ni(s) ? Cr3+(aq) + Ni2+(aq)
A) Cr2O72-(aq) + 3 Ni(s) + 14 H+(aq) ? 2 Cr3+(aq) + 3 Ni2+(aq) + 7 H2O( )
B) Cr2O72-(aq) + Ni(s) + 14 H+(aq) ? 2 Cr3+(aq) + Ni2+(aq) + 7 H2O( ).
C) Cr2O72-(aq) + 3 Ni(s) ? 2 Cr3+(aq) + 3 Ni2+(aq) + O2-(aq)
D) Cr2O72-(aq) + Ni(s) + 14 H+(aq) ? 2 Cr3+(aq) + Ni2+(aq) + 7 H2O( )
E) Cr2O72-(aq) + 3 Ni(s) + 7 H+(aq) ? 2 Cr3+(aq) + 3 Ni2+(aq) + 7 OH-(aq)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following statements is true concerning the voltaic cell shown below? 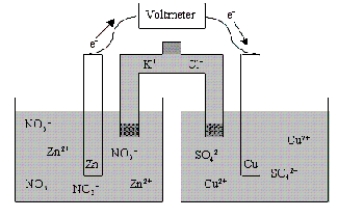
A) The Zn anode mass decreases as the cell discharges.
B) The Zn cathode mass increases as the cell discharges.
C) The Zn cathode mass decreases as the cell discharges.
D) The Zn anode mass increases as the cell discharges.
E) The mass of the Zn electrode neither increases nor decreases as the cell discharges.

A) The Zn anode mass decreases as the cell discharges.
B) The Zn cathode mass increases as the cell discharges.
C) The Zn cathode mass decreases as the cell discharges.
D) The Zn anode mass increases as the cell discharges.
E) The mass of the Zn electrode neither increases nor decreases as the cell discharges.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
21
Write a balanced net ionic equation for the overall reaction represented by the following cell notation. Cu(s) | Cu2+(aq) || Mn2+(aq) | Mn(s)
A) 2 Cu(s) + Mn2+(aq) → Mn(s) + 2 Cu2+(aq)
B) Cu(s) + Cu2+(aq) → Mn(s) + Mn2+(aq)
C) Cu(s) + Mn2+(aq) → Mn(s) + Cu2+(aq)
D) 2 Mn(s) + Cu2+(aq) → Cu(s) + 2Mn2+(aq)
E) Mn(s) + Cu2+(aq) → Cu(s) + Mn2+(aq)
A) 2 Cu(s) + Mn2+(aq) → Mn(s) + 2 Cu2+(aq)
B) Cu(s) + Cu2+(aq) → Mn(s) + Mn2+(aq)
C) Cu(s) + Mn2+(aq) → Mn(s) + Cu2+(aq)
D) 2 Mn(s) + Cu2+(aq) → Cu(s) + 2Mn2+(aq)
E) Mn(s) + Cu2+(aq) → Cu(s) + Mn2+(aq)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
22
Given the following two half-reactions, write the overall reaction in the direction in which it is product-favored, and calculate the standard cell potential.
Pb2+(aq) + 2 e- ? Pb(s)
E° = -0.126 V
Fe3+(aq) + e- ? Fe2+(s)
E° = +0.771 V
A) Pb2+(aq) + 2 Fe2+(s) ? Pb(s) + 2 Fe3+(aq); = +0.897 V
B) Pb2+(aq) + Fe2+(s) ? Pb(s) + Fe3+(aq); = +0.645 V
C) Pb(s) + 2 Fe3+(aq) ? Pb2+(aq) + 2 Fe2+(s); = +1.416 V
D) Pb(s) + 2 Fe3+(aq) ? Pb2+(aq) + 2 Fe2+(s); = +0.897 V
E) Pb(s) + Fe3+(aq) ? Pb2+(aq) + Fe2+(s); = +0.645 V
Pb2+(aq) + 2 e- ? Pb(s)
E° = -0.126 V
Fe3+(aq) + e- ? Fe2+(s)
E° = +0.771 V
A) Pb2+(aq) + 2 Fe2+(s) ? Pb(s) + 2 Fe3+(aq); = +0.897 V
B) Pb2+(aq) + Fe2+(s) ? Pb(s) + Fe3+(aq); = +0.645 V
C) Pb(s) + 2 Fe3+(aq) ? Pb2+(aq) + 2 Fe2+(s); = +1.416 V
D) Pb(s) + 2 Fe3+(aq) ? Pb2+(aq) + 2 Fe2+(s); = +0.897 V
E) Pb(s) + Fe3+(aq) ? Pb2+(aq) + Fe2+(s); = +0.645 V

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
23
Consider the following half-reactions. Ag+(aq) + e- → Ag(s)
E° = +0.80 V
Cu2+(aq) + 2 e- → Cu(s)
E° = +0.34 V
Pb2+(aq) + 2 e- → Pb(s)
E° = -0.13 V
Fe2+(aq) + 2 e- → Fe(s)
E° = -0.44 V
Al3+(aq) + 3 e- → Al(s)
E° = -1.66 V
Which of the following species will oxidize lead, Pb(s)?
A) Ag+(aq) and Cu2+(aq)
B) Ag(s) and Cu(s)
C) Fe2+(aq) and Al3+(aq)
D) Fe(s) and Al(s)
E) Cu2+(aq) and Fe2+(aq)
E° = +0.80 V
Cu2+(aq) + 2 e- → Cu(s)
E° = +0.34 V
Pb2+(aq) + 2 e- → Pb(s)
E° = -0.13 V
Fe2+(aq) + 2 e- → Fe(s)
E° = -0.44 V
Al3+(aq) + 3 e- → Al(s)
E° = -1.66 V
Which of the following species will oxidize lead, Pb(s)?
A) Ag+(aq) and Cu2+(aq)
B) Ag(s) and Cu(s)
C) Fe2+(aq) and Al3+(aq)
D) Fe(s) and Al(s)
E) Cu2+(aq) and Fe2+(aq)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
24
According to the cell notation below, which of the following species is undergoing reduction? Ni | Ni2+(aq) || Mn2+(aq) | MnO2(s) | Pt(s)
A) Mn2+(aq)
B) Ni2+(aq)
C) Ni(s)
D) MnO2(s)
E) Pt(s)
A) Mn2+(aq)
B) Ni2+(aq)
C) Ni(s)
D) MnO2(s)
E) Pt(s)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
25
Use the following standard reduction potentials to determine which species is the best oxidizing agent.
A) I2(s)
B) O2(g)
C) I-(aq)
D) Hg22+(aq)
E) H2O( )
A) I2(s)
B) O2(g)
C) I-(aq)
D) Hg22+(aq)
E) H2O( )

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is the cell notation for a cell in which the hydrogen electrode is the anode and the cathode half-reaction is Co3+(aq) + e− → Co2+(aq)?
A) Pt(s) | H2(g) | H+(aq) || Co3+(aq), Co2+(aq) | Pt(s)
B) Pt(s) | H2(g) | H+(aq) || Co3+(aq), Co2+(aq)
C) Co2+(aq), Co3+(aq) || H+(aq) | H2(g) | Pt(s)
D) Pt(s)| Co2+(aq), Co3+(aq) || H+(aq) | H2(g) | Pt(s)
E) H2(g) | H+(aq) || Co2+(aq), Co3+(aq)
A) Pt(s) | H2(g) | H+(aq) || Co3+(aq), Co2+(aq) | Pt(s)
B) Pt(s) | H2(g) | H+(aq) || Co3+(aq), Co2+(aq)
C) Co2+(aq), Co3+(aq) || H+(aq) | H2(g) | Pt(s)
D) Pt(s)| Co2+(aq), Co3+(aq) || H+(aq) | H2(g) | Pt(s)
E) H2(g) | H+(aq) || Co2+(aq), Co3+(aq)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is the cell notation for a voltaic cell based on the following reaction? Cu2+(aq) + Pb(s) + SO42-(aq) → Cu(s) + PbSO4(s)
A) Pb(s) | PbSO4(s) || Cu2+(aq) || Cu(s)
B) Cu(s) | Cu2+(aq) || SO42-(aq) | PbSO4(s) | Pb(s)
C) Cu(s) | Cu2+(aq), SO42-(aq) | PbSO4(s) | Pb(s)
D) Cu(s) | Cu2+(aq), SO42-(aq) || PbSO4(s) || Pb(s)
E) Pb(s) | PbSO4(s) | SO42-(aq) || Cu2+(aq) || Cu(s)
A) Pb(s) | PbSO4(s) || Cu2+(aq) || Cu(s)
B) Cu(s) | Cu2+(aq) || SO42-(aq) | PbSO4(s) | Pb(s)
C) Cu(s) | Cu2+(aq), SO42-(aq) | PbSO4(s) | Pb(s)
D) Cu(s) | Cu2+(aq), SO42-(aq) || PbSO4(s) || Pb(s)
E) Pb(s) | PbSO4(s) | SO42-(aq) || Cu2+(aq) || Cu(s)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
28
In the given electrochemical cell, which of the following is the cathode half-reaction? Zn(s) | Zn2+(aq) || Fe3+(aq), Fe2+(aq) | Pt(s)
A) Fe3+(aq) + e− → Fe2+(aq)
B) Fe2+(aq) + e− → Fe3+(aq)
C) Fe2+(aq) + Pt(s) → Fe3+(aq) + e−
D) Zn2+(aq) → Zn(s) + 2 e−
E) Zn(s) → Zn2+(aq) + 2 e−
A) Fe3+(aq) + e− → Fe2+(aq)
B) Fe2+(aq) + e− → Fe3+(aq)
C) Fe2+(aq) + Pt(s) → Fe3+(aq) + e−
D) Zn2+(aq) → Zn(s) + 2 e−
E) Zn(s) → Zn2+(aq) + 2 e−

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is the balanced overall reaction and standard cell potential of an electrochemical cell constructed from half-cells with the given half reactions?
Pt2+(aq) + 2 e- ? Pt(s); E° = 1.180 V
Pb2+(aq) + 2 e- ? Pb(s); E° = -0.130 V
A) Pt2+(aq) + Pb(s)? Pt(s) + Pb2+(aq); = 1.310 V
B) Pt(s) + Pb2+(aq) ? Pt2+(aq) + Pb(s); = ?1.310 V
C) Pt2+(aq) + Pb2+(aq) ? Pt(s) + Pb(s); = 1.050 V
D) Pt2+(aq) + Pb(s) ? Pt(s) + Pb2+(aq); =0.655 V
E) Pt(s) + Pb2+(aq) ? Pt2+(aq) + Pb(s); = ?0.655 V
Pt2+(aq) + 2 e- ? Pt(s); E° = 1.180 V
Pb2+(aq) + 2 e- ? Pb(s); E° = -0.130 V
A) Pt2+(aq) + Pb(s)? Pt(s) + Pb2+(aq); = 1.310 V
B) Pt(s) + Pb2+(aq) ? Pt2+(aq) + Pb(s); = ?1.310 V
C) Pt2+(aq) + Pb2+(aq) ? Pt(s) + Pb(s); = 1.050 V
D) Pt2+(aq) + Pb(s) ? Pt(s) + Pb2+(aq); =0.655 V
E) Pt(s) + Pb2+(aq) ? Pt2+(aq) + Pb(s); = ?0.655 V

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
30
Calculate the standard cell potential ( ) for the reaction 2 Ag(s) + Co2+(aq) ? 2 Ag+(aq) + Co(s). The standard reduction potentials are as follows:
Ag+(aq) + e-? Ag(s)
E° = 0.8 V
Co2+(aq) +2 e-? Co(s)
E° = -0.277 V
A) -1.077 V
B) 1.077 V
C) -1.877 V
D) 1.877 V
E) 1.323 V
Ag+(aq) + e-? Ag(s)
E° = 0.8 V
Co2+(aq) +2 e-? Co(s)
E° = -0.277 V
A) -1.077 V
B) 1.077 V
C) -1.877 V
D) 1.877 V
E) 1.323 V

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
31
Write a balanced net ionic equation for the overall reaction represented by the following cell notation. Al(s) | Al3+(aq) || Cl2(g) | Cl-(aq) | Pt(s)
A) 2 Al(s) + 3 Cl2(g) → 2 Al3+(aq) + 6 Cl-(aq)
B) Al(s) + Al3+(aq) → Cl-(aq) + Cl2(g)
C) 2 Al3+(aq) + 6 Cl-(aq) → 2 Al(s) + 3 Cl2(g)
D) Al(s) + 3 Cl2(g) → Al3+(s) + 2 Cl-(aq)
E) Al(s) + 2 Cl-(aq) → Cl2(g) + Al3+(aq)
A) 2 Al(s) + 3 Cl2(g) → 2 Al3+(aq) + 6 Cl-(aq)
B) Al(s) + Al3+(aq) → Cl-(aq) + Cl2(g)
C) 2 Al3+(aq) + 6 Cl-(aq) → 2 Al(s) + 3 Cl2(g)
D) Al(s) + 3 Cl2(g) → Al3+(s) + 2 Cl-(aq)
E) Al(s) + 2 Cl-(aq) → Cl2(g) + Al3+(aq)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
32
Calculate for the electrochemical cell Ag(s) | AgCl(s) | Cl-(aq, 1.0 M) || Cu2+(aq, 1.0 M) | Cu(s). The standard reduction potentials are as follows:
Cu2+(aq) + 2 e- ? Cu(s)
E° = +0.337 V
AgCl(s) + e- ? Ag(s) + Cl-(aq)
E° = +0.222 V
A) -0.115 V
B) -0.107 V
C) +0.115 V
D) +0.452 V
E) +0.559 V
Cu2+(aq) + 2 e- ? Cu(s)
E° = +0.337 V
AgCl(s) + e- ? Ag(s) + Cl-(aq)
E° = +0.222 V
A) -0.115 V
B) -0.107 V
C) +0.115 V
D) +0.452 V
E) +0.559 V

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
33
An SHE electrode has been assigned a standard reduction potential, E°, of 0.00 volts. Which of the following reactions will occur at this electrode?
A) 2 H2O( ) + 2 e- ?H2(g) + 2 OH-(aq)
B) O2(g) + 4 e- ? 2 O2-(aq)
C) Hg2Cl2(s) + 2 e- ? 2 Hg( ) + 2 Cl-(aq)
D) Li+(aq) + e- ? Li(s)
E) 2 H+(aq) + 2 e- ? H2(g)
A) 2 H2O( ) + 2 e- ?H2(g) + 2 OH-(aq)
B) O2(g) + 4 e- ? 2 O2-(aq)
C) Hg2Cl2(s) + 2 e- ? 2 Hg( ) + 2 Cl-(aq)
D) Li+(aq) + e- ? Li(s)
E) 2 H+(aq) + 2 e- ? H2(g)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
34
Consider the following half-reactions. Cl2(g) + 2 e- → 2 Cl-(aq)
E° = +1.36 V
Ag+(aq) + e- → Ag(s)
E° = +0.80 V
Cu2+(aq) + 2 e- → Cu(s)
E° = +0.34 V
Sn2+(aq) + 2 e- → Sn(s)
E° = -0.14 V
Al3+(aq) + 3 e- → Al(s)
E° = -1.66 V
Which of the following species will reduce Cu2+(aq) ion?
A) Ag(s) and Sn2+(aq)
B) Cl-(aq) and Ag(s)
C) Cl2(g) and Ag+(aq)
D) Sn(s) and Al(s)
E) Sn2+(aq) and Al3+(aq)
E° = +1.36 V
Ag+(aq) + e- → Ag(s)
E° = +0.80 V
Cu2+(aq) + 2 e- → Cu(s)
E° = +0.34 V
Sn2+(aq) + 2 e- → Sn(s)
E° = -0.14 V
Al3+(aq) + 3 e- → Al(s)
E° = -1.66 V
Which of the following species will reduce Cu2+(aq) ion?
A) Ag(s) and Sn2+(aq)
B) Cl-(aq) and Ag(s)
C) Cl2(g) and Ag+(aq)
D) Sn(s) and Al(s)
E) Sn2+(aq) and Al3+(aq)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
35
Use the following standard reduction potentials to determine which species is the strongest oxidizing agent.
Fe2+(aq) + 2 e- ? Fe(s); E° = -0.41 V
Pt2+(aq) + 2 e- ? Pt(s); E° = 1.18 V
Cr2O72-(aq) + 14 H+(aq) + 6 e- ? 2 Cr3+(aq) + 7 H2O( ); E° = 1.33 V
A) Fe
B) Pt
C) Cr3+
D) Fe2+
E) Cr2O72-
Fe2+(aq) + 2 e- ? Fe(s); E° = -0.41 V
Pt2+(aq) + 2 e- ? Pt(s); E° = 1.18 V
Cr2O72-(aq) + 14 H+(aq) + 6 e- ? 2 Cr3+(aq) + 7 H2O( ); E° = 1.33 V
A) Fe
B) Pt
C) Cr3+
D) Fe2+
E) Cr2O72-

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
36
Use the following standard reduction potentials to determine which species is the strongest reducing agent. 2 H+(aq) + 2 e- → H2(g); 0.00 V
K+(aq) + e- → K(s); -2.93 V
F2(g) + 2 e- → 2 F-(aq); 2.87 V
Al3+(aq) + 3 e- → Al(s); -1.66 V
Pb2+(aq) + 2 e- → Pb(s); -0.13 V
A) Al3+
B) H+
C) Pb2+
D) F-
E) K
K+(aq) + e- → K(s); -2.93 V
F2(g) + 2 e- → 2 F-(aq); 2.87 V
Al3+(aq) + 3 e- → Al(s); -1.66 V
Pb2+(aq) + 2 e- → Pb(s); -0.13 V
A) Al3+
B) H+
C) Pb2+
D) F-
E) K

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
37
Calculate for the electrochemical cell Pb(s) |PbCl2(s) | Cl-(aq, 1.0 M) || Fe3+(aq, 1.0 M), Fe2+(aq, 1.0 M) | Pt(s). The standard reduction potentials are as follows:
Pb2+(aq) + 2 e- ? Pb(s)
E° = -0.126 V
PbCl2(s) + 2 e- ? Pb(s) + 2 Cl-(aq)
E° = -0.267 V
Fe3+(aq) + e- ? Fe2+(aq)
E° = +0.771 V
Fe2+(aq) + e- ? Fe(s)
E° = -0.44 V
A) -0.504 V
B) -0.062 V
C) +0.504 V
D) +1.038 V
E) +1.604 V
Pb2+(aq) + 2 e- ? Pb(s)
E° = -0.126 V
PbCl2(s) + 2 e- ? Pb(s) + 2 Cl-(aq)
E° = -0.267 V
Fe3+(aq) + e- ? Fe2+(aq)
E° = +0.771 V
Fe2+(aq) + e- ? Fe(s)
E° = -0.44 V
A) -0.504 V
B) -0.062 V
C) +0.504 V
D) +1.038 V
E) +1.604 V

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following species are likely to behave as oxidizing agents? Li(s), H2(g), MnO4-(aq), and Cl-(aq)
A) Li(s)
B) MnO4-(aq)
C) H2(g) and Cl-(aq)
D) Li(s) and MnO4-(aq)
E) Cl-(aq)
A) Li(s)
B) MnO4-(aq)
C) H2(g) and Cl-(aq)
D) Li(s) and MnO4-(aq)
E) Cl-(aq)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
39
The unit for electromotive force, emf, is the volt. 1 volt is equal to _____.
A) one joule per second
B) one coulomb per joule
C) one joule per coulomb
D) one coulomb per second
E) one calorie per second
A) one joule per second
B) one coulomb per joule
C) one joule per coulomb
D) one coulomb per second
E) one calorie per second

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
40
Calculate E°cell for the cell for the reaction 2 Ga(s) + 3 Sn4+(aq) → 3 Sn2+(aq) +2 Ga3+(aq). The standard reduction potentials are as follows:
Ga3+(aq) + 3 e− → Ga(s)
E° = -0.55 V
Sn4+(aq) + 2 e− → Sn2+(aq)
E° = 0.15V
A) 0.70 V
B) 0.40 V
C) 0.33 V
D) -0.40 V
E) −0.70 V
Ga3+(aq) + 3 e− → Ga(s)
E° = -0.55 V
Sn4+(aq) + 2 e− → Sn2+(aq)
E° = 0.15V
A) 0.70 V
B) 0.40 V
C) 0.33 V
D) -0.40 V
E) −0.70 V

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
41
The standard cell potential of the given electrochemical cell is 0.19 V. Pt | Sn4+(aq, 1.0 M), Sn2+(aq, 1.0 M) || Cu2+(aq, 0.200 M) | Cu
Which of the following factors will increase the measured cell potential of the given electrochemical cell?
A) Switching from a platinum to a graphite anode
B) Increasing the size of the anode
C) Decreasing the concentration of Cu2+
D) Increasing the concentration of Sn4+
E) Decreasing the temperature of the cell
Which of the following factors will increase the measured cell potential of the given electrochemical cell?
A) Switching from a platinum to a graphite anode
B) Increasing the size of the anode
C) Decreasing the concentration of Cu2+
D) Increasing the concentration of Sn4+
E) Decreasing the temperature of the cell

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following equations represents the Nernst equation?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
43
For the following cell reaction, the standard cell potential is 1.34 V. To determine the cell potential at nonstandard conditions, what is the value that should be used for n in the Nernst equation?
A) 8
B) 10
C) 5
D) 2
E) 6
A) 8
B) 10
C) 5
D) 2
E) 6

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
44
Calculate the cell potential at 25 °C for the following overall reaction. Zn2+(aq) + 2 Fe2+(aq) → Zn(s) + 2 Fe3+(aq)
[Zn2+] = 1.50 × 10-4 M, [Fe3+] = 0.0200 M, and [Fe2+] = 0.0100 M. The standard reduction potentials are as follows:
Zn2+(aq) + 2 e- → Zn(s)
E° = -0.763 V
Fe3+(aq) + e- → Fe2+(aq)
E° = +0.771 V
A) -1.665 V
B) -1.534 V
C) -1.439 V
D) -0.008 V
E) +0.008 V
[Zn2+] = 1.50 × 10-4 M, [Fe3+] = 0.0200 M, and [Fe2+] = 0.0100 M. The standard reduction potentials are as follows:
Zn2+(aq) + 2 e- → Zn(s)
E° = -0.763 V
Fe3+(aq) + e- → Fe2+(aq)
E° = +0.771 V
A) -1.665 V
B) -1.534 V
C) -1.439 V
D) -0.008 V
E) +0.008 V

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
45
The following has a potential of 0.34 V. If the concentrations of each of the ions is 1.0 M and the pressure of H2 is 1.0 atm, then E° for the half-reaction is _____.
A) 0.17 V
B) -0.17 V
C) 0.34 V
D) -0.34 V
E) None of these
A) 0.17 V
B) -0.17 V
C) 0.34 V
D) -0.34 V
E) None of these

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
46
Calculate Ecell for the following electrochemical cell at 25 °C. Pt(s) | Fe3+(aq, 0.100 M), Fe2+(aq, 0.040 M) || Cl-(aq, 0.50 M) | AgCl(s) | Ag(s)
The standard reduction potentials are as follows:
AgCl(s) + e- → Ag(s) + Cl-(aq)
E° = +0.222 V
Fe3+(aq) + e- → Fe2+(aq)
E° = +0.771 V
A) -1.034 V
B) -0.590 V
C) -0.508 V
D) -0.555 V
E) +1.034 V
The standard reduction potentials are as follows:
AgCl(s) + e- → Ag(s) + Cl-(aq)
E° = +0.222 V
Fe3+(aq) + e- → Fe2+(aq)
E° = +0.771 V
A) -1.034 V
B) -0.590 V
C) -0.508 V
D) -0.555 V
E) +1.034 V

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
47
Calculate Ecell for the following electrochemical cell at 25 °C Pt(s) | H2(g, 1.00 atm) | H+(aq, 1.00 M) || Sn2+(aq, 0.350 M), Sn4+(aq, 0.020 M) | Pt(s)
The standard reduction potentials are as follows:
Sn4+(aq) + 2 e- → Sn2+(s)
E° = +0.15 V
2 H+(aq) + 2 e- → H2(g)
E° = 0.00 V
A) -0.19 V
B) +0.08 V
C) +0.11 V
D) +0.19 V
E) +0.22 V
The standard reduction potentials are as follows:
Sn4+(aq) + 2 e- → Sn2+(s)
E° = +0.15 V
2 H+(aq) + 2 e- → H2(g)
E° = 0.00 V
A) -0.19 V
B) +0.08 V
C) +0.11 V
D) +0.19 V
E) +0.22 V

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
48
For the electrochemical cell Cu(s) | Cu2+ || Ag+ | Ag(s), the standard cell potential is 0.46 V. A cell using these reagents was made, and the observed potential was 0.26 V at 25 oC. Which of the following is a possible explanation for the observed voltage?
A) The volume of the Cu2+ solution was larger than the volume of the Ag+ solution.
B) The volume of the Ag+ solution was larger than the volume of the Cu2+ solution.
C) The Cu2+ concentration was larger than the Ag+ concentration.
D) The Ag electrode was twice as large as the Cu electrode.
E) The Ag+ concentration was larger than the Cu2+ concentration.
A) The volume of the Cu2+ solution was larger than the volume of the Ag+ solution.
B) The volume of the Ag+ solution was larger than the volume of the Cu2+ solution.
C) The Cu2+ concentration was larger than the Ag+ concentration.
D) The Ag electrode was twice as large as the Cu electrode.
E) The Ag+ concentration was larger than the Cu2+ concentration.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following is true for a product-favored reaction at equilibrium?
A) ΔrG° < 0; E°cell > 0
B) ΔrG° > 0; E°cell < 0
C) ΔrG° < 0; E°cell < 0
D) ΔrG° > 0; E°cell > 0
E) ΔrG° > 0; E°cell = 0
A) ΔrG° < 0; E°cell > 0
B) ΔrG° > 0; E°cell < 0
C) ΔrG° < 0; E°cell < 0
D) ΔrG° > 0; E°cell > 0
E) ΔrG° > 0; E°cell = 0

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
50
Calculate the copper(II) ion concentration at 25 °C in the cell Zn(s) | Zn2+(aq, 1.0 M) || Cu2+(aq) | Cu(s) if the measured cell potential is 1.06 V. The standard cell potential is 1.10 V.
A) 0.21 M
B) 1.0 M.
C) 0.045 M
D) 1.0 M..
E) 1.0 M
A) 0.21 M
B) 1.0 M.
C) 0.045 M
D) 1.0 M..
E) 1.0 M

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
51
A voltaic cell or galvanic cell converts chemical energy to electrical energy. for the given galvanic cell is -1.80 V. Fe2+(aq) + 2 Cl-(aq) ? Fe(s) + Cl2(g)
Calculate the value of ?rG° for the reaction.
A) -174 kJ
B) 86.8 kJ
C) 107 kJ
D) 174 kJ
E) 347 kJ
Calculate the value of ?rG° for the reaction.
A) -174 kJ
B) 86.8 kJ
C) 107 kJ
D) 174 kJ
E) 347 kJ

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
52
One Faraday is defined as the:
A) charge on a single electron.
B) quantity of electric charge carried by one mole of electrons.
C) voltage required to reduce one mole of reactant.
D) number of moles of electrons required to reduce one mole of a reactant.
E) charge passed by one ampere of current in one second.
A) charge on a single electron.
B) quantity of electric charge carried by one mole of electrons.
C) voltage required to reduce one mole of reactant.
D) number of moles of electrons required to reduce one mole of a reactant.
E) charge passed by one ampere of current in one second.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
53
The following electrochemical cell has a potential of +0.326 V at 25 °C. Pt | H2(g, 1.00 atm) | H+(aq, 1.00 M) || Cl-(aq) | AgCl(s) | Ag
The standard reduction potential, E°, of AgCl(s) is +0.222 V. Calculate the Cl-(aq) ion concentration.
A) 1.9 × 10-19 M
B) 5.5 × 10-10 M
C) 0.018 M
D) 1.03 M
E) 1.8 × 109 M
The standard reduction potential, E°, of AgCl(s) is +0.222 V. Calculate the Cl-(aq) ion concentration.
A) 1.9 × 10-19 M
B) 5.5 × 10-10 M
C) 0.018 M
D) 1.03 M
E) 1.8 × 109 M

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
54
Calculate ΔrG° for the disproportionation reaction of copper(I) ion (Cu+) at 25 °C. 2 Cu+(aq) → Cu2+(aq) + Cu(s)
The standard reduction potentials are as follows:
Cu+(aq) + e- → Cu(s)
E° = +0.518 V
Cu2+(aq) + 2 e- → Cu(s)
E° = +0.337 V
A) -165 kJ/mol⋅rxn
B) -135 kJ/mol⋅rxn
C) -34.9 kJ/mol⋅rxn
D) +17.5 kJ/mol⋅rxn
E) +135 kJ/mol⋅rxn
The standard reduction potentials are as follows:
Cu+(aq) + e- → Cu(s)
E° = +0.518 V
Cu2+(aq) + 2 e- → Cu(s)
E° = +0.337 V
A) -165 kJ/mol⋅rxn
B) -135 kJ/mol⋅rxn
C) -34.9 kJ/mol⋅rxn
D) +17.5 kJ/mol⋅rxn
E) +135 kJ/mol⋅rxn

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
55
The standard reduction potentials are as follows: Cr3+(aq) + 3 e- → Cr(s); E° = -0.74 V
Fe2+(aq) + 2 e- → Fe(s); E° = -0.41 V
Calculate the standard Gibbs free energy change for the following reaction.
2 Cr(s) + 3 Fe2+ → 3 Fe(s) + 2 Cr3+(aq)
A) 191 kJ
B) 63.7 kJ
C) -504 kJ
D) -191 kJ
E) 1060 kJ
Fe2+(aq) + 2 e- → Fe(s); E° = -0.41 V
Calculate the standard Gibbs free energy change for the following reaction.
2 Cr(s) + 3 Fe2+ → 3 Fe(s) + 2 Cr3+(aq)
A) 191 kJ
B) 63.7 kJ
C) -504 kJ
D) -191 kJ
E) 1060 kJ

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following statements is true concerning the electrochemical cell Zn(s) | Zn2+(aq, 1.0 M) || Ca2+(aq, 1.0 M) | Ca(s) The standard reduction potentials are given follows:
Zn2+(aq) + 2 e− → Zn(s); E° = -0.76 V
Ca2+(aq) + 2 e− → Ca(s); E° = -2.87 V
A) The standard cell potential is -3.63 V.
B) The standard cell potential is -2.11 V.
C) The standard cell potential is -2.11 V..
D) The standard cell potential is -3.63 V..
E) The standard cell potential is 2.11 V.
Zn2+(aq) + 2 e− → Zn(s); E° = -0.76 V
Ca2+(aq) + 2 e− → Ca(s); E° = -2.87 V
A) The standard cell potential is -3.63 V.
B) The standard cell potential is -2.11 V.
C) The standard cell potential is -2.11 V..
D) The standard cell potential is -3.63 V..
E) The standard cell potential is 2.11 V.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
57
If the = -0.362 V for a given electrochemical cell at 25 °C, calculate the pH of the solution at the cathode. Pt | H2(g, 1.0 atm) | H+(aq, 1.00 M) || H+(aq) | H2(g, 1.0 atm) | Pt
A) 1.77
B) 3.06
C) 6.11
D) 7.89
E) 12.23
A) 1.77
B) 3.06
C) 6.11
D) 7.89
E) 12.23

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
58
The cell potential of the following electrochemical cell is determined by using an unspecified concentration of acid. Calculate the pH of the acid solution, given that the measured cell potential is -0.431 V and the anode reduction potential (E°) is 0.222 V at 25 °C.
Ag(s) | AgCl(s) | Cl−(aq, 1.0 M) || H+(aq) | H2(g, 1.0 atm) | Pt(s)
A) 3.53
B) 11.0
C) 4.03
D) 7.06
E) 3.47
Ag(s) | AgCl(s) | Cl−(aq, 1.0 M) || H+(aq) | H2(g, 1.0 atm) | Pt(s)
A) 3.53
B) 11.0
C) 4.03
D) 7.06
E) 3.47

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
59
Calculate the value of the reaction quotient, Q, for the voltaic cell constructed from the following two half-reactions when the Zn2+ion concentration is 0.0110 M and the Ag+ ion concentration is 1.27 M?
? ?
A) 8.66 × 10-3
B) 6.82 × 10-3
C) 115
D) 1.25 × 10-2
E) 147
? ?
A) 8.66 × 10-3
B) 6.82 × 10-3
C) 115
D) 1.25 × 10-2
E) 147

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
60
Calculate Ecell for the following electrochemical cell at 25°C. The standard cell potential, E°cell, is 0.460 V. Cu(s) | Cu2+(aq, 0.016 M) || Ag+(aq, 0.11 M) | Ag(s)
A) 0.456 V
B) 0.282 V
C) 0.460 V
D) 0.485 V
E) 0.452 V
A) 0.456 V
B) 0.282 V
C) 0.460 V
D) 0.485 V
E) 0.452 V

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following are the expected products when an aqueous solution of lithium sulfate is electrolyzed?
A) Li(s) and H2(g)
B) H2(g), OH-(aq), O2(g), and H+(aq)
C) O2(g), H+(aq), and Li(s)
D) H2(g), OH-(aq), and Li(s)
E) H2(g), OH-(aq), and S2O82-(aq)
A) Li(s) and H2(g)
B) H2(g), OH-(aq), O2(g), and H+(aq)
C) O2(g), H+(aq), and Li(s)
D) H2(g), OH-(aq), and Li(s)
E) H2(g), OH-(aq), and S2O82-(aq)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
62
What half-reaction occurs at the cathode during the electrolysis of molten potassium bromide?
A) K(s) → K+(l) + e-
B) Br2(l) + 2 e- → 2 Br-(l)
C) 2 Br-(l) → Br2(l) + 2 e-
D) 2 K+(l) + 2 e- → 2 K(l)
E) 2 H2O(l) + 2 e- → H2(g) + 2 OH-(l)
A) K(s) → K+(l) + e-
B) Br2(l) + 2 e- → 2 Br-(l)
C) 2 Br-(l) → Br2(l) + 2 e-
D) 2 K+(l) + 2 e- → 2 K(l)
E) 2 H2O(l) + 2 e- → H2(g) + 2 OH-(l)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
63
Batteries used in watches contain mercury(II) oxide. As the current flows, mercury(II) oxide is reduced to mercury according to the following reaction: HgO(s) + H2O( ) + 2 e- ? Hg( ) + 2 OH-(aq)
If 2.3 × 10-5 amperes flows continuously for 1200 days, calculate the mass of mercury, Hg( ), produced.
A) 2.5 g
B) 5.0 g
C) 9.9 g
D) 13 g
E) 15 g
If 2.3 × 10-5 amperes flows continuously for 1200 days, calculate the mass of mercury, Hg( ), produced.
A) 2.5 g
B) 5.0 g
C) 9.9 g
D) 13 g
E) 15 g

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
64
Calculate the equilibrium constant for the reaction below at 25 °C. Co(s) + 2 Cr3+(aq) → Co2+(aq) + 2 Cr2+(aq)
The standard reduction potentials are as follows:
Co2+(aq) + 2 e- → Co(s)
E° = -0.28 V
Cr3+(aq) + e- → Cr2+(aq)
E° = -0.41 V
A) 4.0 × 10-5
B) 2.5 × 104
C) 1.0 × 105
D) 1.2 × 105
E) 1.3 × 105
The standard reduction potentials are as follows:
Co2+(aq) + 2 e- → Co(s)
E° = -0.28 V
Cr3+(aq) + e- → Cr2+(aq)
E° = -0.41 V
A) 4.0 × 10-5
B) 2.5 × 104
C) 1.0 × 105
D) 1.2 × 105
E) 1.3 × 105

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
65
How many moles of electrons are produced from a current of 17.0 A in 3.40 hours?
A) 5.99 × 10-4 mol
B) 2.16 mol
C) 57.8 mol
D) 3.35 mol
E) 9.33 × 103 mol
A) 5.99 × 10-4 mol
B) 2.16 mol
C) 57.8 mol
D) 3.35 mol
E) 9.33 × 103 mol

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
66
If ?rG° for the following reaction is -22.2 kJ/mol-rxn, calculate for the following reaction: Cu2+(aq) + 2 Ag(s) + 2 Cl-(aq) ? Cu(s) + 2 AgCl(s)
A) -0.460 V
B) -0.115 V
C) +0.115 V
D) +0.230 V
E) +0.559 V
A) -0.460 V
B) -0.115 V
C) +0.115 V
D) +0.230 V
E) +0.559 V

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
67
When a secondary battery provides electrical energy, it is acting as a voltaic cell. When the battery is recharging, it is operating as a(n) _____ cell.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
68
If an electric current is passed through a solution of molten potassium bromide, KBr, the product at the cathode is _____.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
69
Calculate the equilibrium constant for the following reaction at 25 °C, 2 IO3-(aq) + 5 Hg( ) + 12 H+(aq) ? I2(s) + 5 Hg2+(aq) + 6 H2O( )
The standard reduction potentials are as follows:
IO3-(aq) + 6 H+(aq) + 5 e- ? I2(s) + 3 H2O( )
E° = +1.20 V
Hg2+(aq) + 2 e- ? Hg( )
E° = +0.86 V
A) 3 × 10-58
B) 6 × 105
C) 3 × 1011
D) 6 × 1028
E) 3 × 1057
The standard reduction potentials are as follows:
IO3-(aq) + 6 H+(aq) + 5 e- ? I2(s) + 3 H2O( )
E° = +1.20 V
Hg2+(aq) + 2 e- ? Hg( )
E° = +0.86 V
A) 3 × 10-58
B) 6 × 105
C) 3 × 1011
D) 6 × 1028
E) 3 × 1057

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
70
The value of E°cell is for the following reaction:
Cl2(g) + 2 Fe2+(aq) ? 2 Fe3+(aq) + 2 Cl-(aq)
Calculate the value of E°cell for the reaction below.
Cl-(g) + Fe3+(aq) ? Fe2+(aq) + ½ Cl2(g)
A) -1.18 V
B) -0.30 V
C) 0.59 V
D) 0.30 V
E) -0.59 V
Cl2(g) + 2 Fe2+(aq) ? 2 Fe3+(aq) + 2 Cl-(aq)
Calculate the value of E°cell for the reaction below.
Cl-(g) + Fe3+(aq) ? Fe2+(aq) + ½ Cl2(g)
A) -1.18 V
B) -0.30 V
C) 0.59 V
D) 0.30 V
E) -0.59 V

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
71
Claculate the mass of chromium that can be deposited by electrolysis of an aqueous solution of chromium(III) sulfate, Cr2(SO4)3, for 180 min using a constant current of 11.0 A. Assume 100% current efficiency. (F = 96485 C/mol)
A) 0.356 g
B) 21.3 g
C) 192.1 g
D) 0.187 g
E) 32.0 g
A) 0.356 g
B) 21.3 g
C) 192.1 g
D) 0.187 g
E) 32.0 g

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
72
The standard reduction potentials for a reaction are as follows:
Pb2+(aq) + 2 e- ? Pb(s)
E° = -0.126 V
PbSO4(s) + 2 e- ? Pb(s) + SO42-(aq)
E° = -0.355 V
Calculate the Ksp for lead(II)sulfate (PbSO4) at 25 °C.
A) 3.4 × 10-28
B) 1.8 × 10-8
C) 5.6 × 10-5
D) 5.6 × 107
E) 2.9 × 1037
Pb2+(aq) + 2 e- ? Pb(s)
E° = -0.126 V
PbSO4(s) + 2 e- ? Pb(s) + SO42-(aq)
E° = -0.355 V
Calculate the Ksp for lead(II)sulfate (PbSO4) at 25 °C.
A) 3.4 × 10-28
B) 1.8 × 10-8
C) 5.6 × 10-5
D) 5.6 × 107
E) 2.9 × 1037

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
73
Gold and platinum are commonly used as inert electrodes in laboratory experiments. In commercial applications, such as batteries, _____ is more commonly used as an inert electrodes because it is far less expensive.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
74
Aluminum(III) ion (Al3+) is reduced to solid aluminum at an electrode. If a current of 2.75 amperes is passed for 36 hours, calculate the mass of aluminum deposited at the electrode. (Assume 100% current efficiency.)
A) 9.2 × 10-3 g
B) 3.3 × 101 g
C) 9.9 × 101 g
D) 1.0 × 102 g
E) 3.0 × 102 g
A) 9.2 × 10-3 g
B) 3.3 × 101 g
C) 9.9 × 101 g
D) 1.0 × 102 g
E) 3.0 × 102 g

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
75
The use of electrical energy to produce chemical change is known as _____. An example of this process is the reduction of sodium chloride, NaCl(  ), to produce solid sodium.
), to produce solid sodium.
 ), to produce solid sodium.
), to produce solid sodium.
Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
76
Calculate the value of the equilibrium constant (K) at 25 °C for the following cell reaction: Sn(s) + Pb2+(aq) → Sn2+(aq) + Pb(s); E°cell = 0.014 V
A) 0.014
B) 1.7
C) 0.4
D) 1.0
E) 3
A) 0.014
B) 1.7
C) 0.4
D) 1.0
E) 3

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
77
Calculate the charge, in coulombs, is required to deposit 1.5 g of solid magnesium from a solution of Mg2+(aq) ion.
A) 4.1 × 102 C
B) 6.0 × 103 C
C) 1.2 × 104 C
D) 2.9 × 105 C
E) 3.1 × 106 C
A) 4.1 × 102 C
B) 6.0 × 103 C
C) 1.2 × 104 C
D) 2.9 × 105 C
E) 3.1 × 106 C

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
78
In an electrolytic cell, reduction occurs at the _____ and oxidation occurs at the _____.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
79
Calculate the standard reduction potential for the given reaction at 25 °C. AuCl4-(aq) + 3 e- → Au(s) + 4 Cl-(aq)
The thermodynamic information is as follows:
Au3+(aq) + 3 e- → Au(s)
E° = +1.50 V
Au3+(aq) + 4 Cl-(aq) → AuCl4-(aq)
Kf = 2.3 × 1025
A) -1.28 V
B) -0.50 V
C) +1.00 V
D) +1.28 V
E) +3.85 V
The thermodynamic information is as follows:
Au3+(aq) + 3 e- → Au(s)
E° = +1.50 V
Au3+(aq) + 4 Cl-(aq) → AuCl4-(aq)
Kf = 2.3 × 1025
A) -1.28 V
B) -0.50 V
C) +1.00 V
D) +1.28 V
E) +3.85 V

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
80
A current of 12.0 A is passed through molten magnesium chloride for 14.0 h. How many moles of magnesium metal can be produced from this electrolysis?
A) 0.0522 mol
B) 3.13 mol
C) 0.37 mol
D) 0.22 mol
E) 6.27 mol
A) 0.0522 mol
B) 3.13 mol
C) 0.37 mol
D) 0.22 mol
E) 6.27 mol

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck


