Deck 1: Chemistry - Methods and Measurement
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/104
Play
Full screen (f)
Deck 1: Chemistry - Methods and Measurement
1
Which is the summary of a large amount of scientific information?
A)hypothesis
B)theory
C)scientific law
D)technology
E)scientific method
A)hypothesis
B)theory
C)scientific law
D)technology
E)scientific method
scientific law
2
Which statement concerning the scientific method is FALSE?
A)The scientific method is an organized approach to solving scientific problems.
B)The process of explaining observed behavior begins with a hypothesis.
C)Experimentation is conducted to either support or disprove a hypothesis.
D)A hypothesis becomes a theory when a single experiment supports it.
E)A theory explains scientific observations and data and can help predict new observations and data.
A)The scientific method is an organized approach to solving scientific problems.
B)The process of explaining observed behavior begins with a hypothesis.
C)Experimentation is conducted to either support or disprove a hypothesis.
D)A hypothesis becomes a theory when a single experiment supports it.
E)A theory explains scientific observations and data and can help predict new observations and data.
A hypothesis becomes a theory when a single experiment supports it.
3
1 milligram is equivalent to how many grams?
A)1000
B)100
C)0.1
D)0.01
E)0.001
A)1000
B)100
C)0.1
D)0.01
E)0.001
0.001
4
Which process depicts a physical change?
A)
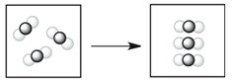
B)
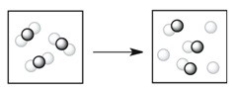
C)
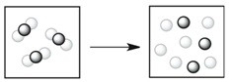
D)
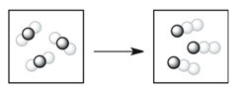
E)None of the processes above depicts a physical change.
A)

B)

C)

D)

E)None of the processes above depicts a physical change.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
5
Which diagram represents a mixture? Note: different colored circles represent atoms of different elements.
A)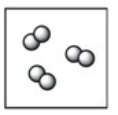
B)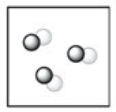
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
6
A patient weighs 146 pounds and is to receive a drug at a dosage of 45.0 mg per kg of body weight.The drug is supplied as a solution that contains 25.0 mg of drug per mL of solution.What volume of the drug should the patient receive? [1 pound = 454 g]
A)0.579 mL
B)119 mL
C)362 mL
D)579 mL
E)119 L
A)0.579 mL
B)119 mL
C)362 mL
D)579 mL
E)119 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
7
If one atom of carbon-14 weighs 14.0 atomic mass units and one atomic mass unit is equal to 1.66 × 10-24 grams,what is the mass of 25 atoms of carbon-14 in grams?
A)5.81 × 10−22
B)5.81 × 10−21
C)581
D)2.11 × 1026
E)2.11 × 10−21
A)5.81 × 10−22
B)5.81 × 10−21
C)581
D)2.11 × 1026
E)2.11 × 10−21

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
8
What type of change alters the appearance,but not the composition or identity of the substance undergoing the change?
A)theoretical
B)physical
C)analytical
D)chemical
E)nuclear
A)theoretical
B)physical
C)analytical
D)chemical
E)nuclear

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
9
When hydrogen (H2)and chlorine (Cl2)gases are mixed,hydrogen chloride (HCl)is produced.Hydrogen chloride is classified as what type of matter?
A)an element
B)a compound
C)a homogeneous mixture
D)a heterogeneous mixture
E)a solution
A)an element
B)a compound
C)a homogeneous mixture
D)a heterogeneous mixture
E)a solution

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
10
Which statement concerning changes in matter is FALSE?
A)A physical change alters the appearance of a substance,but not its identity.
B)A chemical change alters the identity of a substance.
C)A chemical change always results in the production of a new substance.
D)A chemical change is also called a chemical reaction.
E)Melting and freezing are chemical changes that change both the appearance of the substance as well as the identity of the substance.
A)A physical change alters the appearance of a substance,but not its identity.
B)A chemical change alters the identity of a substance.
C)A chemical change always results in the production of a new substance.
D)A chemical change is also called a chemical reaction.
E)Melting and freezing are chemical changes that change both the appearance of the substance as well as the identity of the substance.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
11
What statement best describes an intensive property?
A)A property of a substance that does not depend on the quantity of the substance present.
B)A property of a substance that depends on the quantity of the substance present.
C)A property of a substance that depends on the mass of the substance,but not the volume of the substance.
D)A property of a substance that depends on the physical state (solid,liquid,or gas)of the substance.
E)A property of a substance that changes based on the mass of the material that is present.
A)A property of a substance that does not depend on the quantity of the substance present.
B)A property of a substance that depends on the quantity of the substance present.
C)A property of a substance that depends on the mass of the substance,but not the volume of the substance.
D)A property of a substance that depends on the physical state (solid,liquid,or gas)of the substance.
E)A property of a substance that changes based on the mass of the material that is present.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following terms best describes the sample of matter in the diagram? Note: different colored circles represent atoms of different elements. 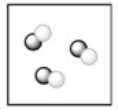
A)homogeneous mixture
B)pure substance
C)heterogeneous mixture
D)solution
E)None of the choices are correct.

A)homogeneous mixture
B)pure substance
C)heterogeneous mixture
D)solution
E)None of the choices are correct.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following terms is most appropriate when classifying an apple?
A)pure substance
B)compound
C)heterogeneous mixture
D)homogeneous mixture
E)solution
A)pure substance
B)compound
C)heterogeneous mixture
D)homogeneous mixture
E)solution

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
14
What is a hypothesis?
A)a fact that results from extensive experimentation and testing
B)the summary of a large quantity of information
C)the result of a single measurement or observation
D)an attempt to explain an observation,or a series of observations
E)an observation of a chemical reaction
A)a fact that results from extensive experimentation and testing
B)the summary of a large quantity of information
C)the result of a single measurement or observation
D)an attempt to explain an observation,or a series of observations
E)an observation of a chemical reaction

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
15
What method used by scientists is the systematic approach to the discovery of new information?
A)analytical method
B)hypothetical method
C)chemical method
D)technological method
E)scientific method
A)analytical method
B)hypothetical method
C)chemical method
D)technological method
E)scientific method

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
16
Which statement concerning the classification of matter is FALSE?
A)All matter is either pure substance or a compound.
B)An element is a pure substance that generally cannot be changed into a simpler form of matter.
C)A compound is a pure substance made up of two or more different elements combined in a definite,reproducible way.
D)A pure substance is composed of only one type of component.
E)A mixture is the physical combination of two or more pure substances in which each substance retains its own identity.
A)All matter is either pure substance or a compound.
B)An element is a pure substance that generally cannot be changed into a simpler form of matter.
C)A compound is a pure substance made up of two or more different elements combined in a definite,reproducible way.
D)A pure substance is composed of only one type of component.
E)A mixture is the physical combination of two or more pure substances in which each substance retains its own identity.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
17
A patient weighs 146 pounds and is to receive a drug at a dosage of 45.0 mg per kg of body weight.What mass of the drug should the patient receive? [1 pound = 454 g]
A)1.47 g
B)2.98 g
C)3.24 mg
D)1470 mg
E)6570 mg
A)1.47 g
B)2.98 g
C)3.24 mg
D)1470 mg
E)6570 mg

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is NOT a type of mixture?
A)homogeneous
B)heterogeneous
C)solution
D)compound
E)All of the choices are correct.
A)homogeneous
B)heterogeneous
C)solution
D)compound
E)All of the choices are correct.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
19
A typical aspirin tablet contains 5.00 grains of pure aspirin analgesic compound.The rest of the tablet is starch.How many aspirin tablets can be made from 50.0 g of pure aspirin? [Use: 1.00 g = 15.4 grains]
A)17 tablets
B)154 tablets
C)250 tablets
D)649 tablets
E)770 tablets
A)17 tablets
B)154 tablets
C)250 tablets
D)649 tablets
E)770 tablets

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
20
A student records the measurement 4.8 m.What type of measurement was made?
A)mass
B)volume
C)length
D)concentration
E)time
A)mass
B)volume
C)length
D)concentration
E)time

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
21
Which temperature would feel the hottest?
A)100°C
B)100°F
C)100 K
D)All temperatures would feel equally hot.
A)100°C
B)100°F
C)100 K
D)All temperatures would feel equally hot.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
22
A bolder at the top of a hill breaks free and rolls down the hill.Which statement best represents the change in energy that occurs in this process?
A)The potential energy of the bolder increases.
B)The potential energy of the bolder is converted to kinetic energy.
C)The kinetic energy of the bolder is converted to potential energy.
D)The chemical energy of the bolder is converted to kinetic energy.
E)No change in energy occurs; energy cannot be converted from one form to another.
A)The potential energy of the bolder increases.
B)The potential energy of the bolder is converted to kinetic energy.
C)The kinetic energy of the bolder is converted to potential energy.
D)The chemical energy of the bolder is converted to kinetic energy.
E)No change in energy occurs; energy cannot be converted from one form to another.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
23
What is the number 3,000 written in scientific notation using the proper number of significant figures?
A)0.003 × 10−3
B)0.3 × 104
C)3 × 103
D)3 × 10−3
E)3.000 × 103
A)0.003 × 10−3
B)0.3 × 104
C)3 × 103
D)3 × 10−3
E)3.000 × 103

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
24
Which statement concerning energy is FALSE?
A)Energy is the amount of heat content in an object.
B)Potential energy is stored energy due to composition or position.
C)Kinetic energy is the energy associated with movement.
D)Heat,light,and electricity are different forms of energy.
E)Conversion of energy from one form to another is possible.
A)Energy is the amount of heat content in an object.
B)Potential energy is stored energy due to composition or position.
C)Kinetic energy is the energy associated with movement.
D)Heat,light,and electricity are different forms of energy.
E)Conversion of energy from one form to another is possible.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following measured volumes has the most uncertainty?
A)10 mL
B)10.0 mL
C)10.00 mL
D)10.000 mL
E)All values have the same degree of uncertainty.
A)10 mL
B)10.0 mL
C)10.00 mL
D)10.000 mL
E)All values have the same degree of uncertainty.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
26
A chemical reaction releases 44.3 kJ of heat.What is the equivalent amount of heat expressed in calories? [1 cal = 4.18 J]
A)10.6 cal
B)106 cal
C)185 cal
D)10,600 cal
E)18,500 cal
A)10.6 cal
B)106 cal
C)185 cal
D)10,600 cal
E)18,500 cal

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
27
The concentration of a patient's blood sugar was determined to be 4850 micrograms per milliliter.Which correctly represents this measurement?
A)4850 μg /ML
B)4850 mg/mL
C)4850 Mg/mL
D)4850 μg/mL
E)4850 mg/ML
A)4850 μg /ML
B)4850 mg/mL
C)4850 Mg/mL
D)4850 μg/mL
E)4850 mg/ML

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
28
What is density?
A)the ratio of the number of particles of a substance to the volume of the solution in which it is dissolved
B)the ratio of the mass of a substance to the volume of the substance
C)the ratio of the volume of a substance to the mass of the substance
D)the ratio of the moles of a substance to the volume of the solution in which it is dissolved
E)the measure of the amount of heat an object contains
A)the ratio of the number of particles of a substance to the volume of the solution in which it is dissolved
B)the ratio of the mass of a substance to the volume of the substance
C)the ratio of the volume of a substance to the mass of the substance
D)the ratio of the moles of a substance to the volume of the solution in which it is dissolved
E)the measure of the amount of heat an object contains

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
29
Air has an average density of 0.001226 g/mL.What volume of air would have a mass of 1.0 lb?
454 g = 1 pound
A)37 mL
B)370 mL
C)557 mL
D)2.7 × 10−6 mL
E)3.7 × 102 L
454 g = 1 pound
A)37 mL
B)370 mL
C)557 mL
D)2.7 × 10−6 mL
E)3.7 × 102 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
30
Where is the uncertainty in the number 101.2°C?
A)in the ones place
B)in the tens place
C)in the tenths place
D)in the hundredths place
E)There is no uncertainty in this number.
A)in the ones place
B)in the tens place
C)in the tenths place
D)in the hundredths place
E)There is no uncertainty in this number.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
31
Which physical property of an astronaut will change depending on whether he or she is on Earth or in orbit?
A)mass
B)weight
C)volume
D)all would change
E)none would change
A)mass
B)weight
C)volume
D)all would change
E)none would change

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
32
If the density of blood is 1.060 g/mL,what is the mass of 6.56 pints of blood?
[1 L = 2.113 pints]
A)3.29 kg
B)329 g
C)2.93 g
D)2930 g
E)2.93 kg
[1 L = 2.113 pints]
A)3.29 kg
B)329 g
C)2.93 g
D)2930 g
E)2.93 kg

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
33
How should the result of the calculation below be reported using scientific notation and the proper number of significant figures?
(4.3169 × 104)÷ (2.02 × 103)= ?
A)2.14 × 101
B)2.1371 × 101
C)2.14 × 102
D)2.14 × 107
E)2.1371 × 109
(4.3169 × 104)÷ (2.02 × 103)= ?
A)2.14 × 101
B)2.1371 × 101
C)2.14 × 102
D)2.14 × 107
E)2.1371 × 109

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
34
A flask contains 145.675 mL of a saline solution.If 24.2 mL of the saline solution are withdrawn from the flask,how should the volume of the saline solution that remains in the flask be reported?
A)121.475 mL
B)121.4 mL
C)121.5 mL
D)122 mL
E)121 mL
A)121.475 mL
B)121.4 mL
C)121.5 mL
D)122 mL
E)121 mL

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
35
What is the number 0.0062985632 written in scientific notation to three significant figures?
A)0.006
B)6.00 × 10−3
C)6.29 × 10−3
D)6.299 × 10−3
E)6.30 × 10−3
A)0.006
B)6.00 × 10−3
C)6.29 × 10−3
D)6.299 × 10−3
E)6.30 × 10−3

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
36
What kind of energy is stored as the result of position or composition?
A)kinetic energy
B)activation energy
C)potential energy
D)theoretical energy
E)static energy
A)kinetic energy
B)activation energy
C)potential energy
D)theoretical energy
E)static energy

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
37
What is the basic unit of volume in the metric system?
A)milliliter
B)cubic centimeter
C)liter
D)gram
E)millimeter
A)milliliter
B)cubic centimeter
C)liter
D)gram
E)millimeter

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
38
What is the number 0.9050 written in scientific notation using the proper number of significant figures?
A)0.9 × 104
B)9 × 10−1
C)9.05 × 10−1
D)9.050 × 104
E)9.050 × 10−1
A)0.9 × 104
B)9 × 10−1
C)9.05 × 10−1
D)9.050 × 104
E)9.050 × 10−1

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
39
A patient needs 0.300 g of a solid drug preparation per day.How many 10.0 mg tablets must be given to the patient per day?
A)3.00 tablets
B)30.0 tablets
C)33.0 tablets
D)300.tablets
E)330.tablets
A)3.00 tablets
B)30.0 tablets
C)33.0 tablets
D)300.tablets
E)330.tablets

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
40
What is the density of a solid object that has the following measurements? mass = 189.6 g,length = 9.80 cm,width = 46.6 mm,height = 0.111 m.
A)0.267 g/mL
B)0.374 g/mL
C)2.67 g/mL
D)3.74 g/mL
E)50.7 g/mL
A)0.267 g/mL
B)0.374 g/mL
C)2.67 g/mL
D)3.74 g/mL
E)50.7 g/mL

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
41
Provide the answer to the following problem using scientific notation and the proper number of significant digits:
(6.00 × 10-2)(3.00 × 10-4)= ?
A)1.8 × 10-5
B)1.80 ×10-5
C)1.80 × 10-4
D)18.00 × 10-4
E)2 × 10-5
(6.00 × 10-2)(3.00 × 10-4)= ?
A)1.8 × 10-5
B)1.80 ×10-5
C)1.80 × 10-4
D)18.00 × 10-4
E)2 × 10-5

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
42
What are the two classes of pure substances?
A)elements and atoms
B)compounds and molecules
C)elements and compounds
D)chemical and physical
E)homogeneous and heterogeneous
A)elements and atoms
B)compounds and molecules
C)elements and compounds
D)chemical and physical
E)homogeneous and heterogeneous

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
43
How many pounds are represented by 764.6 mg?
[1 pound = 454 g]
A)347.1 lb
B)3.471 × 108 lb
C)1.684 × 10-3 lb
D)1.684 lb
E)0.7646 lb
[1 pound = 454 g]
A)347.1 lb
B)3.471 × 108 lb
C)1.684 × 10-3 lb
D)1.684 lb
E)0.7646 lb

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
44
What type of change is represented by the decay of a fallen tree?
A)physical
B)chemical
C)molecular
D)analytical
E)All of the choices are correct.
A)physical
B)chemical
C)molecular
D)analytical
E)All of the choices are correct.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
45
What does the prefix "centi-" mean?
A)10-1
B)10-2
C)10-3
D)102
E)103
A)10-1
B)10-2
C)10-3
D)102
E)103

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
46
In which state does matter have an indefinite shape and definite volume?
A)solid
B)liquid
C)gas
D)All of the choices are correct.
E)None of the choices are correct.
A)solid
B)liquid
C)gas
D)All of the choices are correct.
E)None of the choices are correct.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
47
A student measures the mass of three separate samples of a solid: 104.45 g,0.838 g,and 46 g.If the student mixes all three samples together,how should the total mass be properly reported?
A)151.288
B)151.28
C)151.29
D)151
E)1.5 × 102
A)151.288
B)151.28
C)151.29
D)151
E)1.5 × 102

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
48
Which branch of science primarily involves the study of matter and the changes it undergoes?
A)biology
B)technology
C)physics
D)chemistry
E)All of the choices are correct.
A)biology
B)technology
C)physics
D)chemistry
E)All of the choices are correct.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
49
The green color of the Statue of Liberty is due to a(an)________ change to the copper metal.
A)elemental
B)physical
C)state
D)chemical
E)None of the choices are correct.
A)elemental
B)physical
C)state
D)chemical
E)None of the choices are correct.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
50
What type of property of matter is independent of the quantity of the substance?
A)chemical
B)physical
C)extensive
D)intensive
E)nuclear
A)chemical
B)physical
C)extensive
D)intensive
E)nuclear

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
51
In which state of matter are forces between particles least dominant?
A)solid
B)liquid
C)gas
D)All of the choices are correct.
E)None of the choices are correct.
A)solid
B)liquid
C)gas
D)All of the choices are correct.
E)None of the choices are correct.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
52
Conversion of ice to liquid water or liquid water to steam is an example of what kind of change?
A)physical
B)chemical
C)molecular
D)analytical
E)Both physical and chemical are correct.
A)physical
B)chemical
C)molecular
D)analytical
E)Both physical and chemical are correct.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
53
What term is used to describe the summary of a large quantity of information?
A)hypothesis
B)theory
C)law
D)model
E)result
A)hypothesis
B)theory
C)law
D)model
E)result

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
54
How many significant figures does the number 5.06305 × 104 contain?
A)4
B)5
C)6
D)7
E)9
A)4
B)5
C)6
D)7
E)9

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
55
Which measurement represents the largest volume?
A)4.6 L
B)4.6 × 10−3 L
C)46 cL
D)460 mL
E)All represent the same volume.
A)4.6 L
B)4.6 × 10−3 L
C)46 cL
D)460 mL
E)All represent the same volume.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
56
How many centimeters correspond to 15.68 kilometers?
A)1.568 × 106 cm
B)1.568 × 105 cm
C)1.568 × 10-4 cm
D)1568 cm
E)1.569 cm
A)1.568 × 106 cm
B)1.568 × 105 cm
C)1.568 × 10-4 cm
D)1568 cm
E)1.569 cm

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following terms is defined as anything that has mass and occupies space?
A)chemistry
B)element
C)matter
D)compound
E)volume
A)chemistry
B)element
C)matter
D)compound
E)volume

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
58
The cost of a drug is 125 francs per gram.What is the cost in dollars per ounce?
[$1 = 6.25 francs and 1 ounce = 28.4 g]
A)$0.70/oz
B)$568/oz
C)$27.5/oz
D)$2.22 × 104/oz
E)$4.65/oz
[$1 = 6.25 francs and 1 ounce = 28.4 g]
A)$0.70/oz
B)$568/oz
C)$27.5/oz
D)$2.22 × 104/oz
E)$4.65/oz

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
59
If a person smokes 10.0 packs of cigarettes a week and each cigarette contains 5.00 mg of tar,how many years will she have to smoke to inhale 0.250 pounds of tar?
[20 cigarettes = 1 pack,1 pound = 454 g and 1 year = 52 weeks]
A)2.18 y
B)2.18 × 10-2 y
C)1.06 y
D)28.6 y
E)0.556 y
[20 cigarettes = 1 pack,1 pound = 454 g and 1 year = 52 weeks]
A)2.18 y
B)2.18 × 10-2 y
C)1.06 y
D)28.6 y
E)0.556 y

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
60
Which state of matter has neither a definite shape nor a definite volume?
A)liquid
B)solid
C)gas
D)vapor
E)Both gas and vapor are correct.
A)liquid
B)solid
C)gas
D)vapor
E)Both gas and vapor are correct.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following is an example of physical change?
A)boiling water
B)burning paper
C)a metal losing electrons to become a cation
D)cooking eggs
E)lighting a match
A)boiling water
B)burning paper
C)a metal losing electrons to become a cation
D)cooking eggs
E)lighting a match

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
62
Which one of the following is an example of an extensive property?
A)density
B)specific gravity
C)mass
D)hardness
E)boiling temperature
A)density
B)specific gravity
C)mass
D)hardness
E)boiling temperature

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
63
Round 0.052018 to three significant figures.
A)0.05
B)0.052
C)0.0520
D)0.05201
E)0.05202
A)0.05
B)0.052
C)0.0520
D)0.05201
E)0.05202

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
64
What Kelvin temperature corresponds to 98.6°F?
A)310 K
B)310.2 K
C)31.00 K
D)132.0 K
E)199 K
A)310 K
B)310.2 K
C)31.00 K
D)132.0 K
E)199 K

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following is FALSE concerning the gas state?
A)Gases have no definite shape.
B)Gases have no definite volume.
C)Particles are far apart from each other.
D)Particles are usually in a regular or organized pattern.
E)When gas molecules collide,they do not lose energy.
A)Gases have no definite shape.
B)Gases have no definite volume.
C)Particles are far apart from each other.
D)Particles are usually in a regular or organized pattern.
E)When gas molecules collide,they do not lose energy.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
66
Air is a/an
A)element.
B)compound.
C)mixture.
D)molecule.
E)pure substance.
A)element.
B)compound.
C)mixture.
D)molecule.
E)pure substance.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following is a chemical property?
A)flammability
B)color
C)hardness
D)temperature
E)melting point
A)flammability
B)color
C)hardness
D)temperature
E)melting point

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
68
What Celsius temperature corresponds to -4.6°F?
A)-20°C
B)-20.3°C
C)-23.0°C
D)-10.9°C
E)-68.4°C
A)-20°C
B)-20.3°C
C)-23.0°C
D)-10.9°C
E)-68.4°C

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
69
Which temperature scale does not use a degree sign?
A)Celsius
B)Kelvin
C)Centigrade
D)Fahrenheit
E)Absolute zero
A)Celsius
B)Kelvin
C)Centigrade
D)Fahrenheit
E)Absolute zero

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
70
The speed of light is 186,000 miles per second.What is its speed in centimeters per second?
[5280 feet = 1 mile; 12 inches = 1 foot; 2.54 cm = 1 inch]
A)3.01 × 1011 cm/s
B)3.15 × 1010 cm/s
C)6.06 × 1012 cm/s
D)3 × 1011 cm/s
E)2.99 × 1010 cm/s
[5280 feet = 1 mile; 12 inches = 1 foot; 2.54 cm = 1 inch]
A)3.01 × 1011 cm/s
B)3.15 × 1010 cm/s
C)6.06 × 1012 cm/s
D)3 × 1011 cm/s
E)2.99 × 1010 cm/s

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
71
Select the answer that best expresses the result of the following calculation:
1.86 + 246.4 - 79.9208 = ?
A)168
B)168.3
C)168.34
D)168.339
E)168.3392
1.86 + 246.4 - 79.9208 = ?
A)168
B)168.3
C)168.34
D)168.339
E)168.3392

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
72
What kind of change always results in the formation of new materials?
A)molecular
B)exothermic
C)endothermic
D)physical
E)chemical
A)molecular
B)exothermic
C)endothermic
D)physical
E)chemical

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
73
What is the appropriate number of significant figures necessary to express the result of the calculation below?
(51.6)× (3.1416)
A)1
B)2
C)3
D)4
E)5
(51.6)× (3.1416)
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
74
The distance between two hydrogen atoms in a hydrogen molecule (H2)is 7.461 × 10-11m.What is the equivalent distance expressed in inches?
[2.54 cm = 1 in]
A)2 × 10-9 in
B)1.895 × 10-12 in
C)294 × 10-11 in
D)2.937 × 10-9 in
E)2.94 × 10-8 in
[2.54 cm = 1 in]
A)2 × 10-9 in
B)1.895 × 10-12 in
C)294 × 10-11 in
D)2.937 × 10-9 in
E)2.94 × 10-8 in

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
75
What Fahrenheit temperature corresponds to -40.0°C?
A)-8°F
B)16.8°F
C)-36.9°F
D)-40.0°F
E)-1.94°F
A)-8°F
B)16.8°F
C)-36.9°F
D)-40.0°F
E)-1.94°F

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
76
What is the specific gravity of an object that weighs 13.35 g and has a volume of 25.00 mL? The density of water under the same conditions is 0.980 g/mL.
A)1.335
B)0.545 g/mL
C)0.534 g/mL
D)0.545
E)0.980
A)1.335
B)0.545 g/mL
C)0.534 g/mL
D)0.545
E)0.980

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
77
1 centimeter equals how many millimeters?
A)10-6
B)10-3
C)10
D)104
E)106
A)10-6
B)10-3
C)10
D)104
E)106

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
78
Which one of the following is an example of a pure substance?
A)ethanol
B)sugar water
C)salt and pepper
D)milk
E)sand
A)ethanol
B)sugar water
C)salt and pepper
D)milk
E)sand

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following is NOT a physical property of matter?
A)odor
B)compressibility
C)flash point
D)melting point
E)color
A)odor
B)compressibility
C)flash point
D)melting point
E)color

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
80
If the density of carbon tetrachloride is 1.59 g/mL,what is the volume in L,of 4.21 kg of carbon tetrachloride?
A)0.149 L
B)0.378 L
C)2.65 L
D)6.69 L
E)6690 L
A)0.149 L
B)0.378 L
C)2.65 L
D)6.69 L
E)6690 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck


