Deck 16: Aldehydes and Ketones
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/114
Play
Full screen (f)
Deck 16: Aldehydes and Ketones
1
What is the condensed formula of the compound shown below? 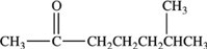
A)CH3CO(CH2)3CH(CH3)2
B)CH3OC(CH2)3CHCH3CH3
C)CH3CO(CH2)3CHCH3CH3
D)CH3OCCH2CH2CH2CH2CH(CH3)2
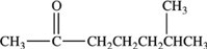
A)CH3CO(CH2)3CH(CH3)2
B)CH3OC(CH2)3CHCH3CH3
C)CH3CO(CH2)3CHCH3CH3
D)CH3OCCH2CH2CH2CH2CH(CH3)2
CH3CO(CH2)3CH(CH3)2
2
What product is formed when the compound below is oxidized with K2Cr2O7? 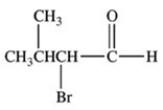
A)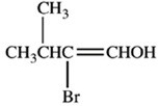
B)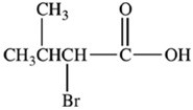
C)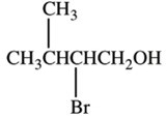
D)No reaction occurs.

A)

B)

C)

D)No reaction occurs.

3
The compound shown below belongs to what class of compounds? 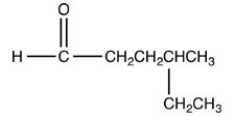
A)Alkane
B)Aldehyde
C)Ketone
D)Acetal
E)Carboxylic acid

A)Alkane
B)Aldehyde
C)Ketone
D)Acetal
E)Carboxylic acid
Aldehyde
4
What is the structure of 1-chloro-3-ethyl-2-heptanone?
A)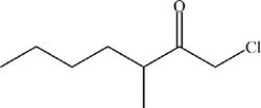
B)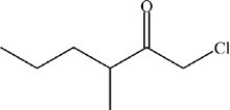
C)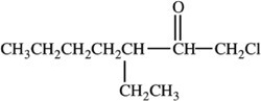
D)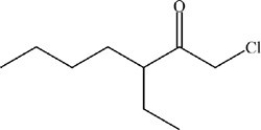
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
5
The Tollens reagent is which of the following?
A)K2Cr2O7
B)H2SO4
C)Cl2
D)Ag2O in aqueous NH4OH
A)K2Cr2O7
B)H2SO4
C)Cl2
D)Ag2O in aqueous NH4OH

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
6
The compound shown below belongs to what class of compounds? 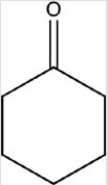
A)Alkane
B)Aldehyde
C)Ketone
D)Acetal
E)Carboxylic acid

A)Alkane
B)Aldehyde
C)Ketone
D)Acetal
E)Carboxylic acid

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
7
What is the common name of the compound shown below? 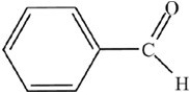
A)Benzene aldehyde
B)Benzaldehyde
C)Acetaldehyde
D)Phenone

A)Benzene aldehyde
B)Benzaldehyde
C)Acetaldehyde
D)Phenone

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following represents the general condensed formula for an aldehyde?
A)RCOOH
B)RCHO
C)RCOR
D)RCOH
E)RCH2OH
A)RCOOH
B)RCHO
C)RCOR
D)RCOH
E)RCH2OH

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
9
Which compound has the greatest solubility in water?
A)CH3(CH2)5CHO
B)CH3(CH2)4COCH2CH3
C)CH3CH2CHO
D)The compounds all are equally soluble in water.
A)CH3(CH2)5CHO
B)CH3(CH2)4COCH2CH3
C)CH3CH2CHO
D)The compounds all are equally soluble in water.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
10
What is the IUPAC name of the compound shown below? 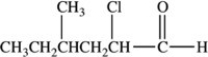
A)5-chloro-3-methylhexanal
B)2-chloro-4-methylhexanal
C)5-chloro-3-methyl-6-hexanal
D)2-chloro-4-methylhexanone
E)3-chloro-5-methylhexanone
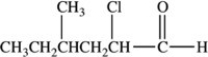
A)5-chloro-3-methylhexanal
B)2-chloro-4-methylhexanal
C)5-chloro-3-methyl-6-hexanal
D)2-chloro-4-methylhexanone
E)3-chloro-5-methylhexanone

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
11
Which compound is an example of a ketone?
A)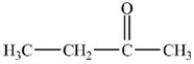
B)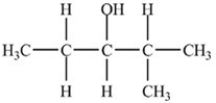
C)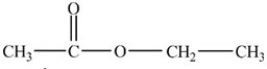
D)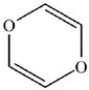
E)None of the structures are ketones.
A)
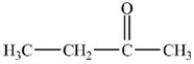
B)

C)
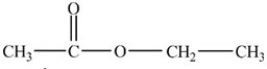
D)

E)None of the structures are ketones.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
12
Which compound is soluble in heptane,but not soluble in water?
A)(CH3)2CH(CH2)5CHO
B)CH3COCH3
C)CH3CH2CHO
D)All of the compounds are soluble in both heptane and water.
A)(CH3)2CH(CH2)5CHO
B)CH3COCH3
C)CH3CH2CHO
D)All of the compounds are soluble in both heptane and water.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
13
Which compound has the highest boiling point?
A)CH3(CH2)5CH3
B)CH3(CH2)4CHO
C)CH3(CH2)4CH2OH
D)All of the compounds have the same boiling point.
A)CH3(CH2)5CH3
B)CH3(CH2)4CHO
C)CH3(CH2)4CH2OH
D)All of the compounds have the same boiling point.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
14
Which compound will give a positive result in a Tollens test?
A)
B)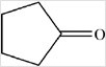
C)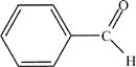
D)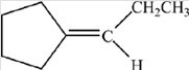
E)More than one of the compounds will give a positive Tollens test result.
A)

B)

C)

D)

E)More than one of the compounds will give a positive Tollens test result.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
15
Which compound(s)would give a positive Tollens test?
A)Alcohols
B)Aldehydes
C)Ketones
D)Carboxylic acids
E)Alcohols and aldehydes
A)Alcohols
B)Aldehydes
C)Ketones
D)Carboxylic acids
E)Alcohols and aldehydes

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
16
What is the IUPAC name of the compound shown below? 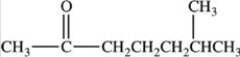
A)Methyl hexyl ketone
B)6,6-dimethyl-2-hexanone
C)6-methyl-2-heptanone
D)2-methyl-6-heptanone
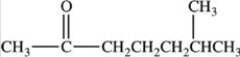
A)Methyl hexyl ketone
B)6,6-dimethyl-2-hexanone
C)6-methyl-2-heptanone
D)2-methyl-6-heptanone

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
17
Which is the structure of 3-bromo-2-methylbutanal?
A)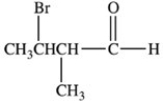
B)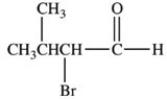
C)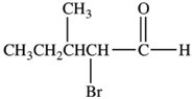
D)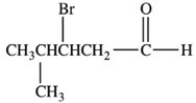
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
18
What is the IUPAC name of the compound shown below? 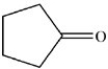
A)1-pentanone
B)Cyclopentanone
C)Phenone
D)Cyclopentone

A)1-pentanone
B)Cyclopentanone
C)Phenone
D)Cyclopentone

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
19
What is the structure of 2-ethyl-4-methyloctanal?
A)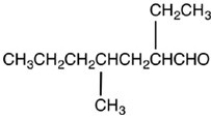
B)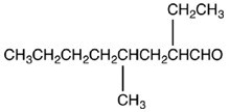
C)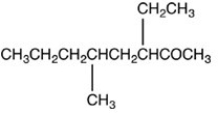
D)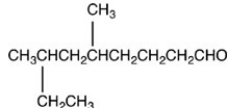
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
20
Which compound could be oxidized to form the carboxylic acid below? 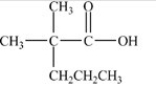
A)CH3(CH2)5CHO
B)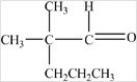
C)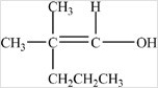
D)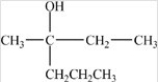

A)CH3(CH2)5CHO
B)

C)

D)


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
21
What hemiacetal is formed in the reaction below? 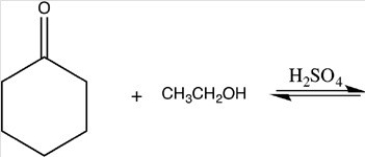
A)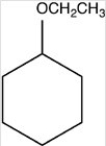
B)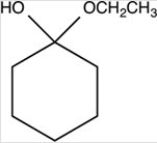
C)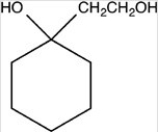
D)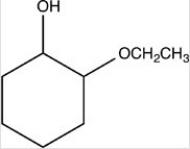

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
22
What is the acetal formed in the reaction below,if two equivalents of alcohol are used? 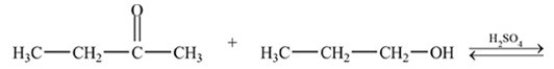
A)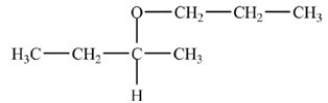
B)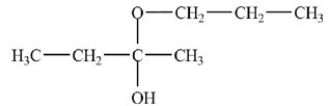
C)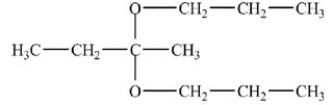
D)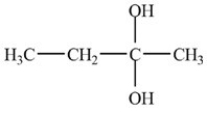

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
23
What product is formed when the following molecule is treated with H2 and a Pd catalyst? 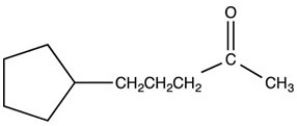
A)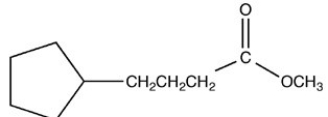
B)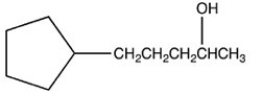
C)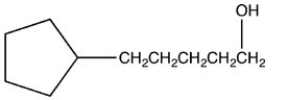
D)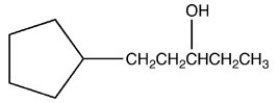

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
24
Which reactant would be needed to form the alcohol product CH3CH2CH2OH by a reduction reaction?
A)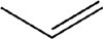
B)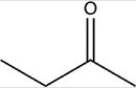
C)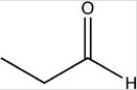
D)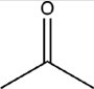
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
25
What hemiacetal is formed in the reaction below? 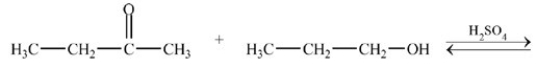
A)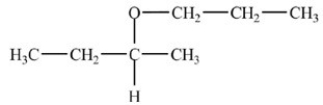
B)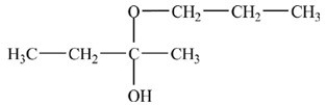
C)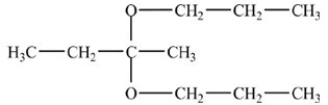
D)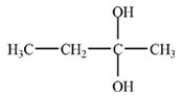
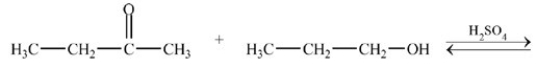
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
26
Which compound is a hemiacetal?
A)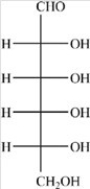
B)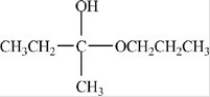
C)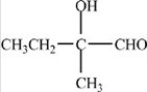
D)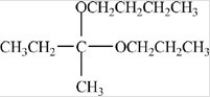
E)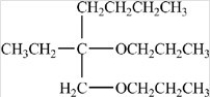
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
27
What reaction does the coenzyme NADH,in the presence of an enzyme,facilitate?
A)The reduction of a carbonyl group to form a carboxylic acid
B)The reduction of a carbonyl group to form an alcohol
C)The reduction of a carboxylic acid to form a carbonyl group
D)The oxidation of a carbonyl group to form an alcohol
E)The oxidation of a carbonyl group to form a carboxylic acid
A)The reduction of a carbonyl group to form a carboxylic acid
B)The reduction of a carbonyl group to form an alcohol
C)The reduction of a carboxylic acid to form a carbonyl group
D)The oxidation of a carbonyl group to form an alcohol
E)The oxidation of a carbonyl group to form a carboxylic acid

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
28
Which compound is an acetal?
A)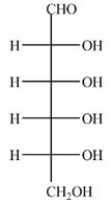
B)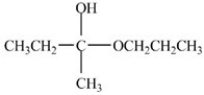
C)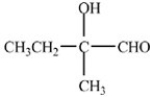
D)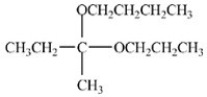
E)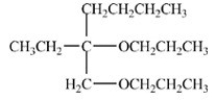
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
29
What product is formed when the compound shown below is treated with Tollens reagent? 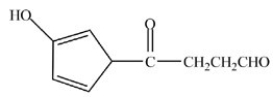
A)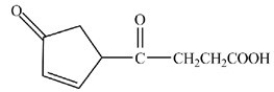
B)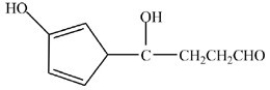
C)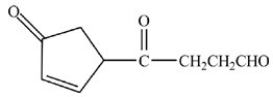
D)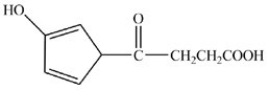

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
30
The compound below is classified as what type of compound? 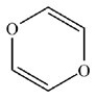
A)An acetal
B)A hemiacetal
C)An ether
D)An acetal and an ether

A)An acetal
B)A hemiacetal
C)An ether
D)An acetal and an ether

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
31
The compound below is classified as what type of compound? 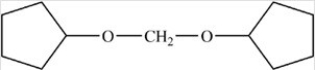
A)An acetal
B)A hemiacetal
C)An ether
D)A cyclic acetal
E)An acetal and an ether

A)An acetal
B)A hemiacetal
C)An ether
D)A cyclic acetal
E)An acetal and an ether

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
32
What is the product of the reduction of a ketone?
A)A primary alcohol
B)A secondary alcohol
C)A tertiary alcohol
D)A carboxylic acid
E)An ether
A)A primary alcohol
B)A secondary alcohol
C)A tertiary alcohol
D)A carboxylic acid
E)An ether

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
33
Which reactant would be needed to form the alcohol product shown below by a reduction reaction? 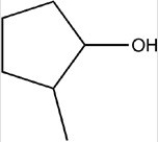
A)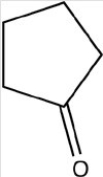
B)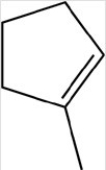
C)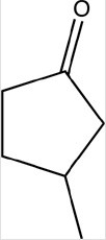
D)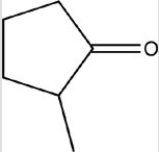

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
34
What product is formed when the following molecule is treated with H2 and a Pd catalyst? 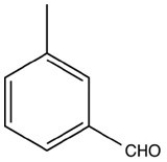
A)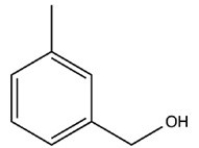
B)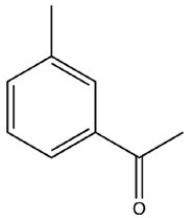
C)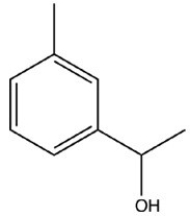
D)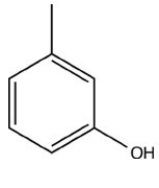

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
35
What hemiacetal is formed when the hydroxyl aldehyde shown below is cyclized? 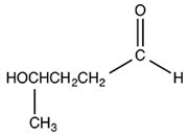
A)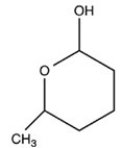
B)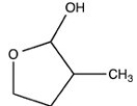
C)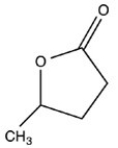
D)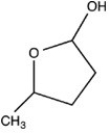

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
36
What acetal is formed when the compound shown below is treated with two equivalents of ethanol (CH3CH2OH)in the presences of H2SO4? 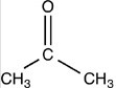
A)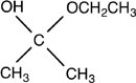
B)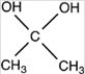
C)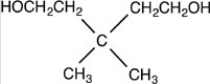
D)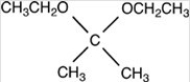

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
37
What is the product of the reduction of an aldehyde?
A)A primary alcohol
B)A secondary alcohol
C)A tertiary alcohol
D)A carboxylic acid
E)An ether
A)A primary alcohol
B)A secondary alcohol
C)A tertiary alcohol
D)A carboxylic acid
E)An ether

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
38
What products are formed when the acetal below is hydrolyzed with water and sulfuric acid? 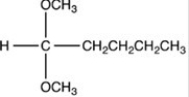
A)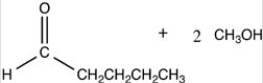
B)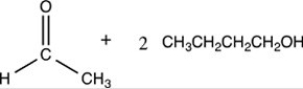
C)HOCH2CH2CH2CH2CH3 + 2 CH3OH
D)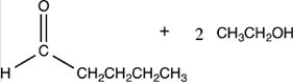

A)
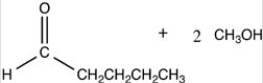
B)

C)HOCH2CH2CH2CH2CH3 + 2 CH3OH
D)


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
39
A hemiacetal contains which two groups bonded to the same carbon atom?
A)An OH (hydroxyl)group and an OR (alkoxy)group
B)Two OH (hydroxyl)groups
C)Two OR (alkoxy)groups
D)An OH (hydroxyl)group and a C=O (carbonyl)group
A)An OH (hydroxyl)group and an OR (alkoxy)group
B)Two OH (hydroxyl)groups
C)Two OR (alkoxy)groups
D)An OH (hydroxyl)group and a C=O (carbonyl)group

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
40
Which reagent can be used to reduce a ketone?
A)K2Cr2O7
B)H2SO4
C)H2 with Pd metal
D)Ag2O in aqueous NH4OH
A)K2Cr2O7
B)H2SO4
C)H2 with Pd metal
D)Ag2O in aqueous NH4OH

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
41
The following ketones play an important role in the tanning industry and are found in many commercial sunscreens. Which ketone is expected to be the most soluble in water and therefore the most readily washed off when an individual goes swimming?
A)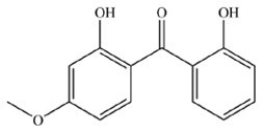
B)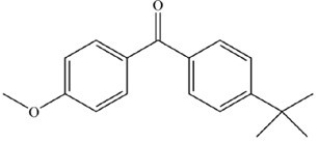
C)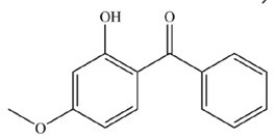
D)All would be expected to have the same solubility.
A)

B)

C)

D)All would be expected to have the same solubility.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
42
What product results when cinnamaldehyde is reduced with excess H2 in the presence of a Pd catalyst? 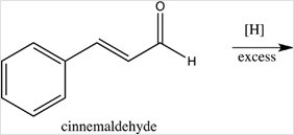
A)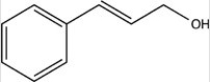
B)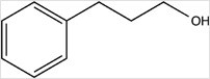
C)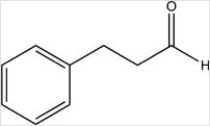
D)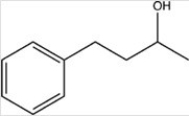

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
43
What is the IUPAC name of the compound below? 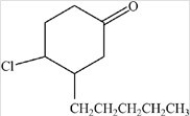
A)4-chloro-3-pentyl-1-cyclohexone
B)4-chloro-3-pentylcyclohexanone
C)1-chloro-2-pentyl-4-cyclohexanone
D)4-chloro-3-pentylphenone

A)4-chloro-3-pentyl-1-cyclohexone
B)4-chloro-3-pentylcyclohexanone
C)1-chloro-2-pentyl-4-cyclohexanone
D)4-chloro-3-pentylphenone

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
44
The compound butanone does not need a number to specify the location of the carbonyl group on the carbon chain. Which statement best explains why?
A)The C=O is assumed to be on C-1 of the carbon chain if it is not specified.
B)The C=O can only be on C-2 of the carbon chain in this compound.
C)The location of the C=O does not need to be specified for a cyclic ketone.
D)The C=O is on an end carbon of the carbon chain.
A)The C=O is assumed to be on C-1 of the carbon chain if it is not specified.
B)The C=O can only be on C-2 of the carbon chain in this compound.
C)The location of the C=O does not need to be specified for a cyclic ketone.
D)The C=O is on an end carbon of the carbon chain.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
45
The compound decanal contributes to the odor and flavor of an orange. Which of the statements concerning the structure of decanal is incorrect?
A)Decanal is an aldehyde.
B)Decanal contains a saturated chain of ten carbons.
C)Decanal contains a carbonyl group on the first carbon of the carbon chain.
D)Decanal is an aromatic compound.
A)Decanal is an aldehyde.
B)Decanal contains a saturated chain of ten carbons.
C)Decanal contains a carbonyl group on the first carbon of the carbon chain.
D)Decanal is an aromatic compound.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
46
What hydrolysis products are formed when the acetal (CH3CH2CH2)2C(OCH2CH3)2 is treated with H2SO4 in H2O?
A)(CH3CH2CH2)2CHOCH2CH3 + CH3CH2OH
B)(CH3CH2CH2)2CO + 2 CH3CH2OH
C)(CH3CH2CH2)2CHOH + 2 CH3CH2OH
D)CH2(OCH2CH3)2 + 2 CH3CH2CH3
A)(CH3CH2CH2)2CHOCH2CH3 + CH3CH2OH
B)(CH3CH2CH2)2CO + 2 CH3CH2OH
C)(CH3CH2CH2)2CHOH + 2 CH3CH2OH
D)CH2(OCH2CH3)2 + 2 CH3CH2CH3

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
47
Deoxyribose is a building block of DNA. Which statement concerning the functional groups present in deoxyribose,shown below,is incorrect? 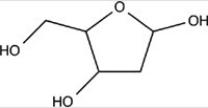
A)It contains an acetal.
B)It contains an alcohol.
C)It contains a hemiacetal.
D)None of these choices are incorrect.

A)It contains an acetal.
B)It contains an alcohol.
C)It contains a hemiacetal.
D)None of these choices are incorrect.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
48
What is the name of the compound below? 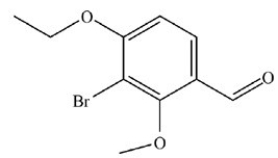
A)3-bromo-4-ethyl-2-methylbenzaldehyde
B)3-bromo-4-ethoxy-2-methoxybenzaldehyde
C)1-bromo-2-ethoxy-6-methoxy-3-benzaldehyde
D)3-bromo-4-ethoxy-2-methoxyphenal

A)3-bromo-4-ethyl-2-methylbenzaldehyde
B)3-bromo-4-ethoxy-2-methoxybenzaldehyde
C)1-bromo-2-ethoxy-6-methoxy-3-benzaldehyde
D)3-bromo-4-ethoxy-2-methoxyphenal

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
49
What type of compound is shown below? 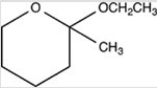
A)An epoxide
B)A cyclic hemiacetal
C)An aldehyde
D)A cyclic acetal

A)An epoxide
B)A cyclic hemiacetal
C)An aldehyde
D)A cyclic acetal

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
50
What is the structure of 2-bromo-5-hydroxyheptanal?
A)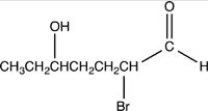
B)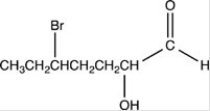
C)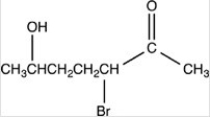
D)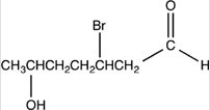
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
51
Salicin is a naturally occurring pain reliever isolated from the bark of willow trees. Which statement concerning the functional groups present in salicin is incorrect? 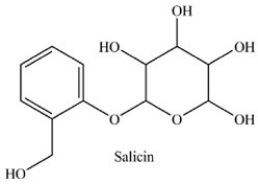
A)It contains an acetal.
B)It contains an alcohol.
C)It contains a hemiacetal.
D)It contains a phenol.

A)It contains an acetal.
B)It contains an alcohol.
C)It contains a hemiacetal.
D)It contains a phenol.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following is the product of the reduction of acetone,shown below? 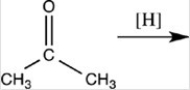
A)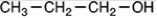
B)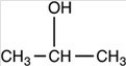
C)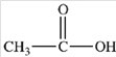
D)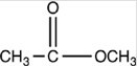

A)
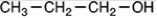
B)

C)

D)


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
53
What product results when cinnamaldehyde is oxidized? 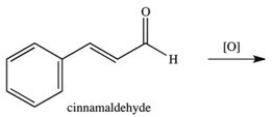
A)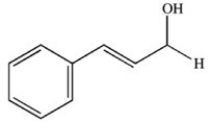
B)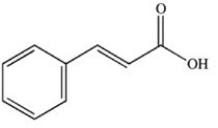
C)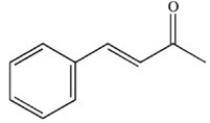
D)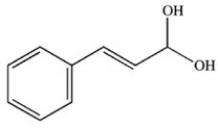

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
54
In the correct order,what are the three reactions that convert all-trans-retinal into 11-cis-retinal so that the process of vision can continue?
A)Reduction,Oxidation,Isomerization
B)Reduction,Isomerization,Oxidation
C)Oxidation,Isomerization,Reduction
D)Oxidation,Reduction,Isomerization
A)Reduction,Oxidation,Isomerization
B)Reduction,Isomerization,Oxidation
C)Oxidation,Isomerization,Reduction
D)Oxidation,Reduction,Isomerization

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
55
What is the name of the compound below? 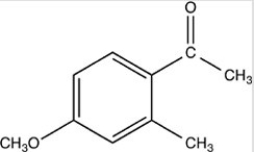
A)1-methoxy-3-methylbenzene
B)4-methoxy-6-methylbenzaldehyde
C)4-methoxy-2-methylacetophenone
D)5-methoxy-3-methyltoluene

A)1-methoxy-3-methylbenzene
B)4-methoxy-6-methylbenzaldehyde
C)4-methoxy-2-methylacetophenone
D)5-methoxy-3-methyltoluene

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
56
What type of compound is shown below? 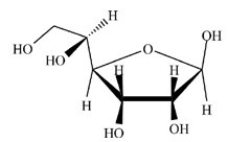
A)An epoxide
B)A cyclic hemiacetal
C)An aldehyde
D)A cyclic acetal

A)An epoxide
B)A cyclic hemiacetal
C)An aldehyde
D)A cyclic acetal

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
57
What is the name of the compound below? 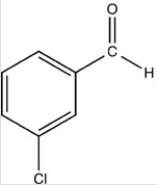
A)3-chlorobenzenal
B)3-methanalchlorobenzene
C)M-chlorobenzaldehyde
D)P-chloroacetophenone

A)3-chlorobenzenal
B)3-methanalchlorobenzene
C)M-chlorobenzaldehyde
D)P-chloroacetophenone

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
58
What is the name of the compound below? 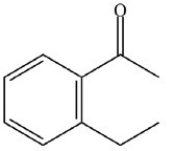
A)2-ethylbenzenone
B)2-ethylacetophenone
C)2-ethylacetobenzone
D)2-ethylphenone

A)2-ethylbenzenone
B)2-ethylacetophenone
C)2-ethylacetobenzone
D)2-ethylphenone

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
59
What is the structure of 3-hydroxy-2-methylbenzaldehyde?
A)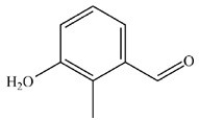
B)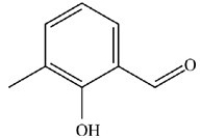
C)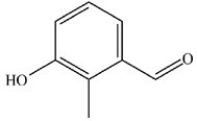
D)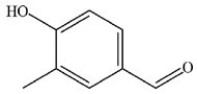
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
60
The reduction of acetaldehyde is an important biochemical reaction. Which of the following is the product of its reduction? 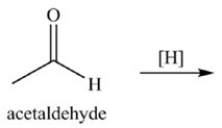
A)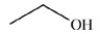
B)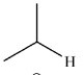
C)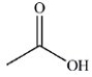
D)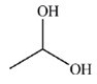

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
61
The reaction below illustrates oxidation of an aldehyde. 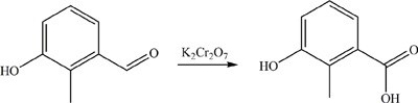


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
62
When light hits the retina,the 11-cis double bond of 11-cis-retinal is isomerized to its more stable trans isomer,and the all-trans-retinal is formed.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
63
The reaction below illustrates oxidation by Tollens reagent. 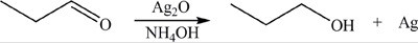


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
64
Ketones are not oxidized since they contain no H atom bonded to the carbonyl carbon.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
65
Vanillin,acetone,and formaldehyde are aldehydes.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
66
The reaction below illustrates oxidation by Tollens' reagent. 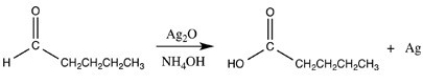


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
67
When the hemiacetal below reacts with ethanol,what acetal is formed? 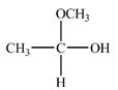
A)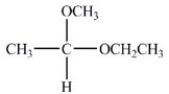
B)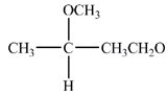
C)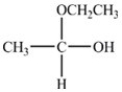
D)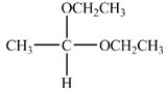

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
68
The structure below contains both an acetal and hemiacetal. 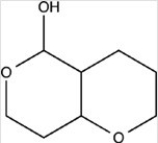


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
69
Triamcinolone acetonide (structure shown),a synthetic corticosteroid drug,contains an acetal. 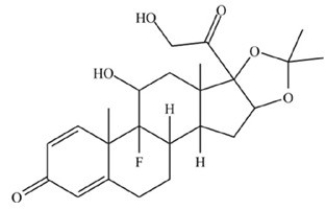


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
70
4,4-Dimethyl-2-hexanone is a ketone of molecular formula C8H16O that has six carbons in its longest chain.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
71
The carbohydrate glucose exists predominantly as a cyclic hemiacetal.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
72
11-cis-retinal is a ketone in the rod cells of the eye that is critical for vision.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
73
Acetals are stable molecules,but they can be converted back to aldehydes and ketones by treatment with acid and water.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
74
Acetals are ethers.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
75
Cyclic hemiacetals are converted to acetals by reaction with an alcohol.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
76
Aldehydes and ketones undergo addition reactions with alcohols (ROH)to form hemiacetals and acetals.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
77
Aldehydes and ketones undergo addition reactions with alcohols (ROH)to form hemiacetals and acetals.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
78
As is in the laboratory,the reduction of carbonyl groups in biological systems is accomplished using H2 and Pd as a reducing agent.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
79
D-Glucose,shown below,will give a positive Tollens test. 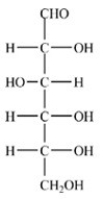


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
80
The compound below is an acetal. 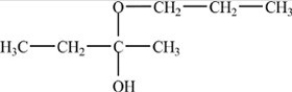


Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck


