Deck 8: Elimination Reactions: the E1 and E2 Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/45
Play
Full screen (f)
Deck 8: Elimination Reactions: the E1 and E2 Reactions
1
What will happen to the rate of the reaction below if the concentration of the alkyl chloride is doubled and the concentration of the sodium ethoxide is cut in half? 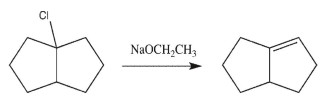
A)The rate will double.
B)The rate will decrease by a factor of two.
C)The rate will quadruple.
D)The rate will decrease by a factor of four.
E)The rate will remain the same.

A)The rate will double.
B)The rate will decrease by a factor of two.
C)The rate will quadruple.
D)The rate will decrease by a factor of four.
E)The rate will remain the same.
The rate will remain the same.
2
Which of the following carbocations is (are) most likely to rearrange? 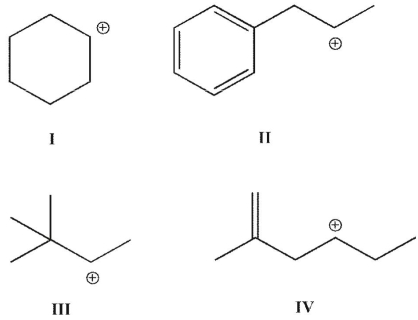
A) I
B) II
C) III
D) II, III, and IV
E) I, II and IV

A) I
B) II
C) III
D) II, III, and IV
E) I, II and IV
II, III, and IV
3
What will happen to the rate of the reaction below if the concentration of the bromide is tripled? 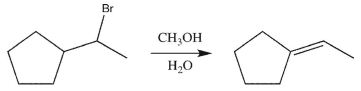
A) The rate will double.
B) The rate will be cut in half.
C) The rate will decrease by a factor of one-third.
D) The rate will increase by a factor of nine.
E) The rate will triple.

A) The rate will double.
B) The rate will be cut in half.
C) The rate will decrease by a factor of one-third.
D) The rate will increase by a factor of nine.
E) The rate will triple.
The rate will triple.
4
Which is the most likely product of the reaction shown here? 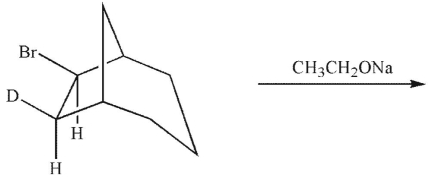
A)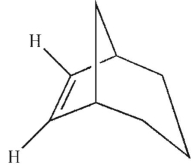
B)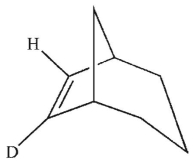
C)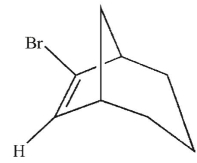
D)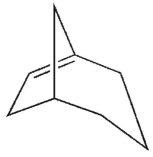
E)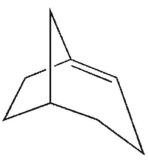

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
5
What is the major SN1 product of the following reaction?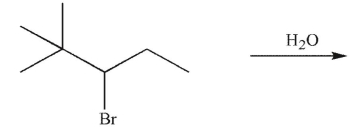
A)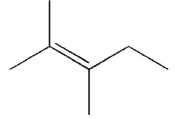
B)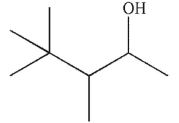
C)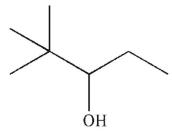
D)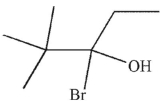
E)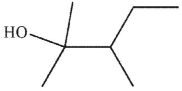

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
6
Ehats the major E1 product of this reaction ?
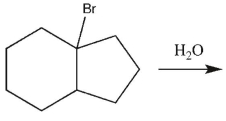
A)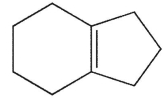
B)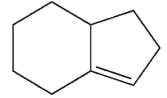
C)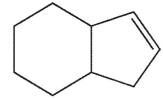
D)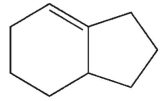
E)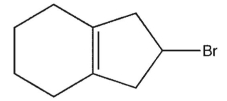

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
7
Which of these is the most stable carbanion? 
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
8
What will happen to the rate of the reaction below if the concentration of ethanol is doubled?
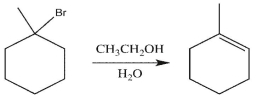
A) The rate will double.
B) The rate will be cut in half.
C) The rate will be unaffected.
D) The rate will increase by a factor of four.
E) The rate will decrease by a factor of four.

A) The rate will double.
B) The rate will be cut in half.
C) The rate will be unaffected.
D) The rate will increase by a factor of four.
E) The rate will decrease by a factor of four.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
9
What is the major E1 product of the reaction ?
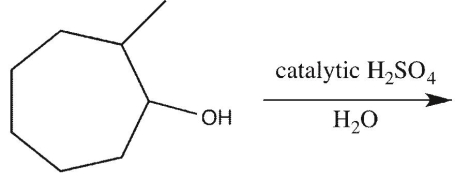
A)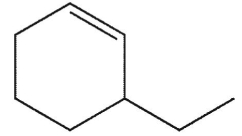
B)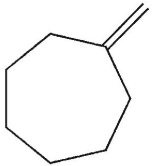
C)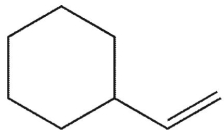
D)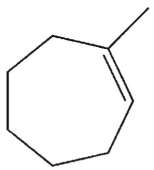
E)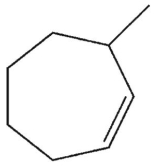

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements is true?
A)The formation of the kinetic product has a higher activation energy than the formation of the thermodynamic product.
B)The kinetic product is more stable than the thermodynamic product.
C)The thermodynamic product forms faster than the kinetic product.
D)The transition state leading to the kinetic product is lower in energy than those leading to alternative products.
E)The kinetic and thermodynamic products form at the same rate; it is the energy difference between them that determines product distribution.
A)The formation of the kinetic product has a higher activation energy than the formation of the thermodynamic product.
B)The kinetic product is more stable than the thermodynamic product.
C)The thermodynamic product forms faster than the kinetic product.
D)The transition state leading to the kinetic product is lower in energy than those leading to alternative products.
E)The kinetic and thermodynamic products form at the same rate; it is the energy difference between them that determines product distribution.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following intermediates has the lowest activation energy for its formation, assuming identical leaving groups? 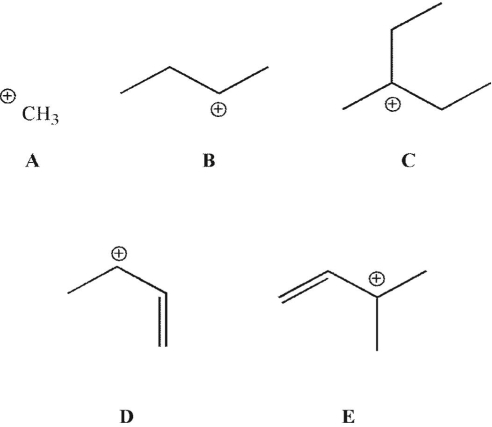
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following carbocations is least likely to rearrange? 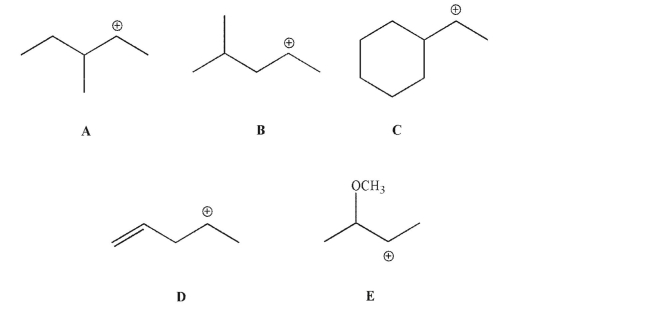
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
13
Which is the least likely product of the reaction shown here? 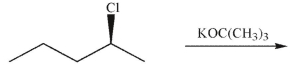
A)
B)
C)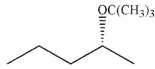
D)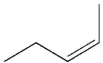
E) All four products are equally likely to form .

A)

B)

C)

D)

E) All four products are equally likely to form .

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
14
What is the major product of this reaction? 
A)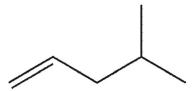
B)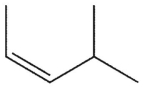
C)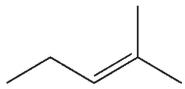
D)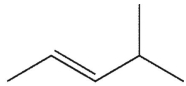
E)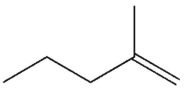

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
15
In which of these reactions do you expect the structure of the transition state to most closely resemble the structure of the product? 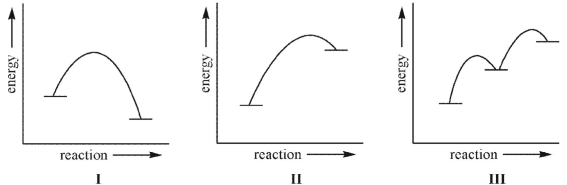
A) I
B) II
C) III
D) II and III
E) I, II, and III

A) I
B) II
C) III
D) II and III
E) I, II, and III

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
16
What will happen to the rate of the reaction below if the concentration of potassium tert-butoxide is doubled? 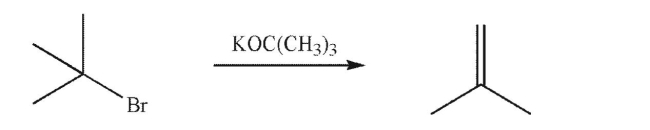
A)The rate will double.
B)The rate will triple.
C)The rate will quadruple.
D)The rate will increase by a factor of 1.5.
E)The rate will remain the same.

A)The rate will double.
B)The rate will triple.
C)The rate will quadruple.
D)The rate will increase by a factor of 1.5.
E)The rate will remain the same.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
17
Which of these structures shows the preferred conformation for an E2 reaction to occur?
A)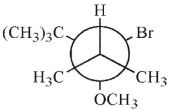
B)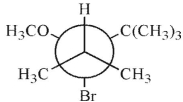
C)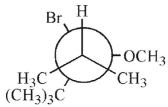
D)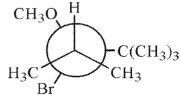
E)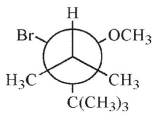
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
18
Consider the energy diagram for the multistep reaction shown here.Which statement about this diagram is not true? 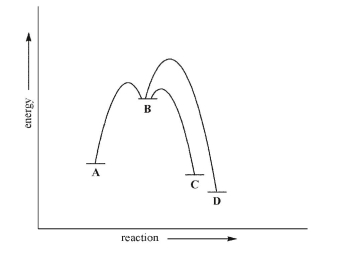
A)C is the kinetic product.
B)D is the thermodynamic product.
C)Given sufficient energy, C and D can equilibrate.
D)Formation of D is under thermodynamic control.
E)Formation of C is under thermodynamic control.

A)C is the kinetic product.
B)D is the thermodynamic product.
C)Given sufficient energy, C and D can equilibrate.
D)Formation of D is under thermodynamic control.
E)Formation of C is under thermodynamic control.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
19

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following statements is true about the two processes shown here? 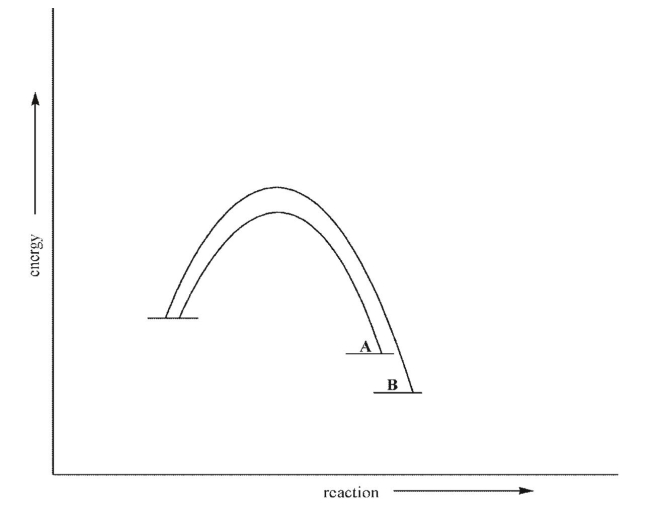
A)A forms faster than B because A is more stable.
B)B forms faster than A because B is more stable.
C)A forms faster than B because the transition state leading to A is lower in energy than the transition state leading to B.
D)B forms faster than A because the transition state leading to B is lower in energy than the transition state leading to A.
E)A and B form at the same rate.

A)A forms faster than B because A is more stable.
B)B forms faster than A because B is more stable.
C)A forms faster than B because the transition state leading to A is lower in energy than the transition state leading to B.
D)B forms faster than A because the transition state leading to B is lower in energy than the transition state leading to A.
E)A and B form at the same rate.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
21
Use the Hammond postulate to predict which of the following processes will be faster, and to
explain the difference in rates of formation of carbocation A and carbocation B.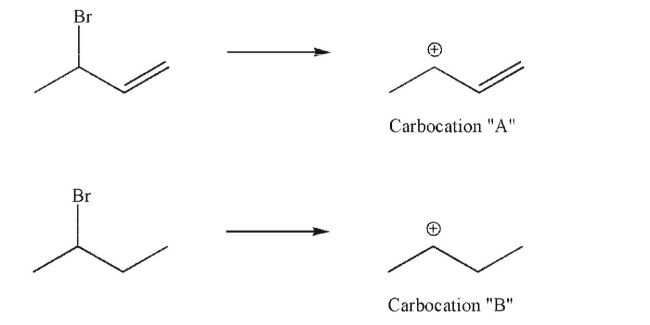
explain the difference in rates of formation of carbocation A and carbocation B.


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
22
Draw the product of the following E2 reaction.
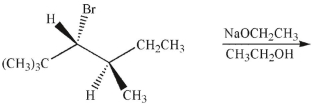


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
23
State the Hammond postulate.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
24
In the presence of aqueous sulfuric acid, (E)-3-methyl-2-pentene can be protonated at two
different carbons to provide two different carbocation intermediates.Draw one energy diagram
showing both possible protonation events.Include in your diagram
a.the product of each of these protonation steps,
b.the relative energies of the transition states leading to these products, and
c.the relative energies of these products.
different carbons to provide two different carbocation intermediates.Draw one energy diagram
showing both possible protonation events.Include in your diagram
a.the product of each of these protonation steps,
b.the relative energies of the transition states leading to these products, and
c.the relative energies of these products.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
25
Draw the major organic product of the following reaction. 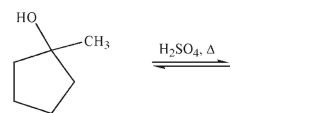


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
26
Draw a mechanism to rationalize the formation of the alkene product shown in the following
transformation.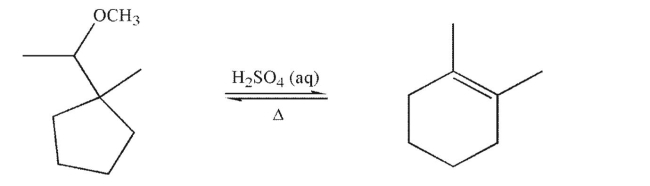
transformation.


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
27
Draw a mechanism to rationalize the formation of the alkene product shown in the following
transformation.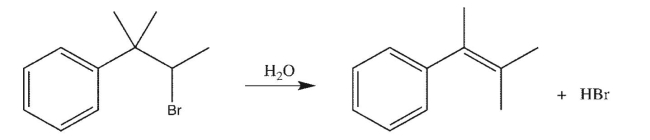
transformation.


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
28
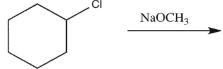
Explain why Reaction A would give more E2 product than Reaction B, while Reaction B would give more SN2 product than Reaction A.
Reaction A:
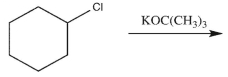
Reaction B:
Reaction A:

Reaction B:


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is the product of the reaction conditions shown? 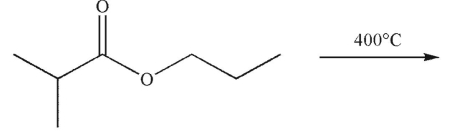
A)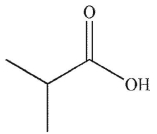
B)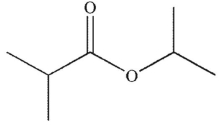
C)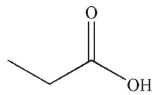
D)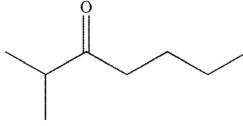
E)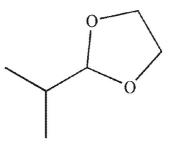

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
30
Draw the product of the following E2 reaction and provide a mechanism for its formation.


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
31
Draw the substitution product that would result from each reaction below.Indicate which reaction
would occur faster and provide an explanation for your answer using any necessary diagrams and
structures as support.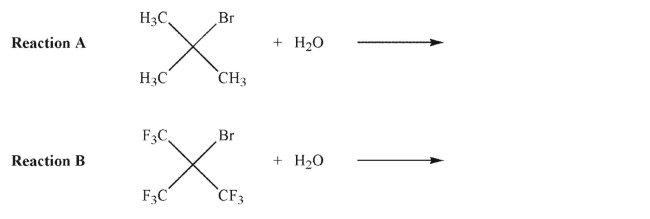
would occur faster and provide an explanation for your answer using any necessary diagrams and
structures as support.


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following reactions would give relatively more E2 than SN2 product?
Reaction A:
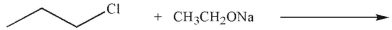
Reaction B:
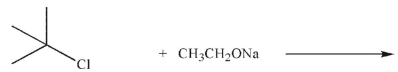
Reaction A:
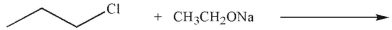
Reaction B:
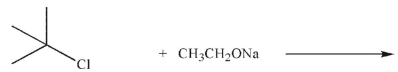

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following structures is not a possible product of the reaction shown here? 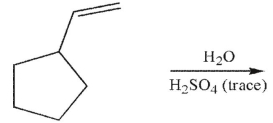
A)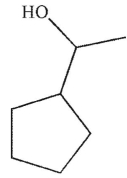
B)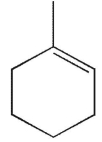
C)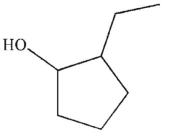
D)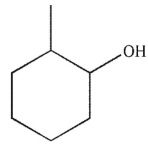
E)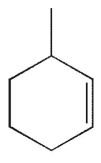

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
34
The reaction shown here is the pinacol rearrangement.It uses patterns of reactivity and
mechanism you have already seen.Provide a mechanism to rationalize the formation of the
product shown in this reaction.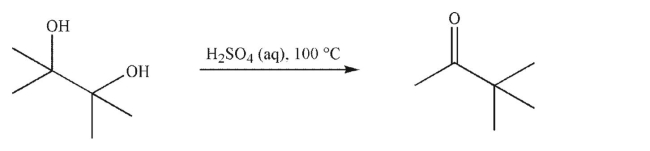
mechanism you have already seen.Provide a mechanism to rationalize the formation of the
product shown in this reaction.


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
35
Draw the major El product of the following reaction.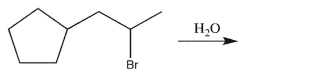


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
36
Suppose that in the E1 reaction shown here-the dehydration of cyclopentanol-a nonpolar
solvent like hexane was used instead of water.What would be the effect on the rate of reaction
compared to using water as solvent, and why?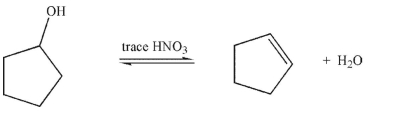
solvent like hexane was used instead of water.What would be the effect on the rate of reaction
compared to using water as solvent, and why?


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
37
Draw the product of the following E2 reaction and provide a mechanism for its formation.
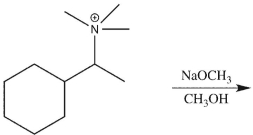


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
38
Draw the structures of all possible El and SN1 products that can form under the conditions shown.
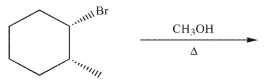


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
39
Draw the product of the following E2 reaction. Show stereochemistry at all stereogenic carbons.
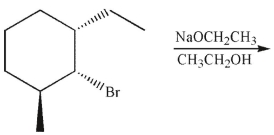


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
40
Explain the difference between kinetic control and thermodynamic control.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
41
Predict the product of the following reaction conditions and draw a mechanism to rationalize its
formation.Include all necessary lone pairs of electrons, curved arrows, and nonzero formal
charges.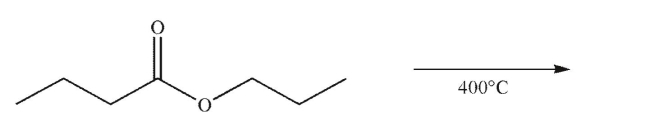
formation.Include all necessary lone pairs of electrons, curved arrows, and nonzero formal
charges.


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
42
Draw a mechanism to account for the formation of the product shown here. 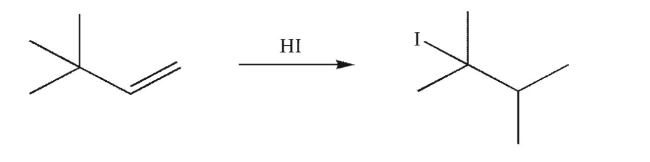


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
43
Draw all the possible addition products formed in the reaction shown. 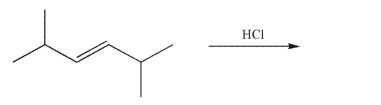


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
44
Propose a mechanism to account for the formation of the observed product. 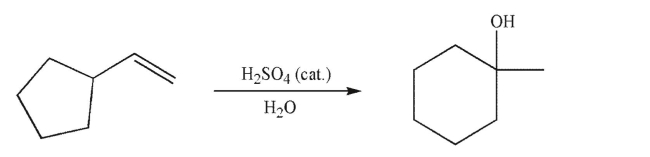


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
45
Propose a mechanism to account for the formation of the observed product. 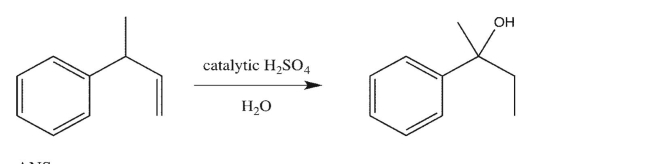


Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck


