Deck 13: Dienes and the Allyl System: 2p Orbitals in Conjugation
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/68
Play
Full screen (f)
Deck 13: Dienes and the Allyl System: 2p Orbitals in Conjugation
1
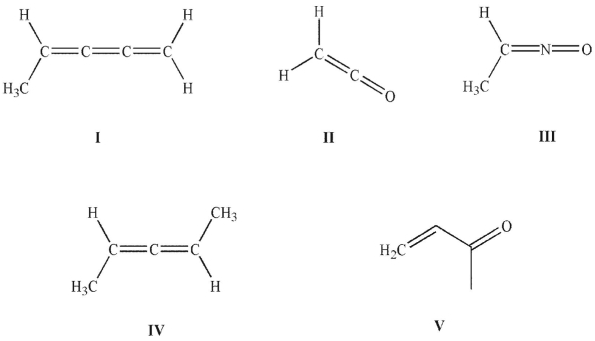
Which of the following structures is a ketene?
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V
II
2
From left to right, what is the approximate hybridization of the indicated carbons in the structure shown here?
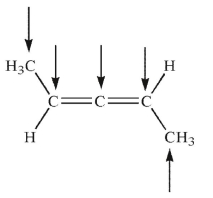
A)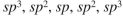
B)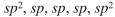
C)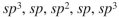
D)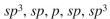
E)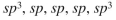

A)
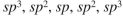
B)
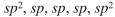
C)
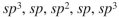
D)
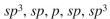
E)
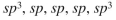
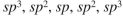
3
Which of the following compounds is the thermodynamic product of the reaction shown here? 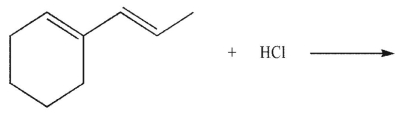
A)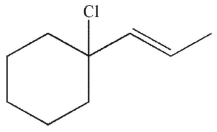
B)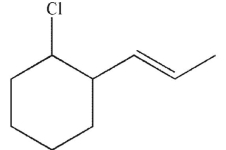
C)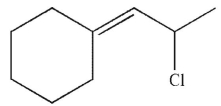
D)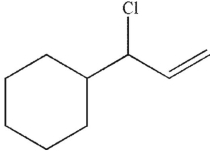
E)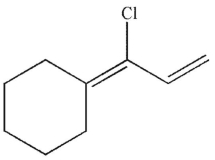

A)

B)

C)

D)

E)


4
When 1,3 -butadiene reacts with chlorine, a 50/50 mixture of 3,4 -dichloro-1-butene and (E)-1,4-dichloro-2-butene is generated. When the same diene is reacted with bromine, the 3,4 -dibromo-1-butene is the predominant product.. What could explain the difference in regioselectivity?
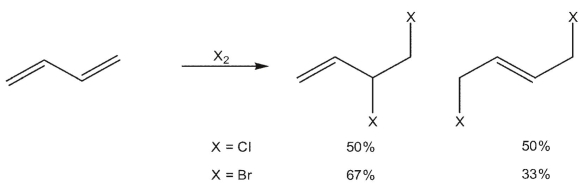
A) The more stable intermediate bromonium ion favors 1,2 -addition.
B) The more reactive bromide anion favors the kinetic product.
C) The more reactive chloride anion favors the thermodynamic product.
D) The more stable intermediate chloronium ion favors the 1,4 -addition product.
E) The electronegative bromine destabilizes the positive charge on the β-carbon.

A) The more stable intermediate bromonium ion favors 1,2 -addition.
B) The more reactive bromide anion favors the kinetic product.
C) The more reactive chloride anion favors the thermodynamic product.
D) The more stable intermediate chloronium ion favors the 1,4 -addition product.
E) The electronegative bromine destabilizes the positive charge on the β-carbon.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following molecules contains the most acidic hydrogen?
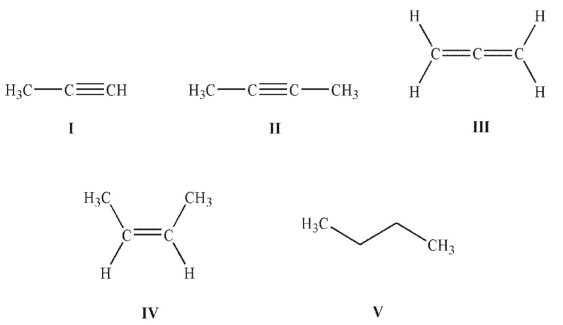
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
6
How many nodes are there in the highest energy 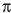 molecular orbital for 1,3,5 -hexatriene?
molecular orbital for 1,3,5 -hexatriene?
A) 0
B) 2
C) 3
D) 5
E) 6
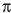 molecular orbital for 1,3,5 -hexatriene?
molecular orbital for 1,3,5 -hexatriene?A) 0
B) 2
C) 3
D) 5
E) 6

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following statements about the reaction shown here is true? 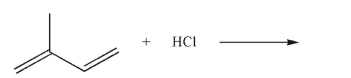
A)The major product under kinetic conditions is the 1,2 addition product because of the stability of the carbocation intermediate in that pathway.
B)The major product under kinetic conditions is the 1,2 addition product because of proximity effects.
C)The major product under kinetic conditions is the 1,4 addition product because of the stability of the carbocation intermediate in that pathway.
D)The major product under kinetic conditions is the 1,4 addition product because of proximity effects.
E)Neither carbocation stability nor proximity effects play a role in determining the preferred product under kinetic conditions.

A)The major product under kinetic conditions is the 1,2 addition product because of the stability of the carbocation intermediate in that pathway.
B)The major product under kinetic conditions is the 1,2 addition product because of proximity effects.
C)The major product under kinetic conditions is the 1,4 addition product because of the stability of the carbocation intermediate in that pathway.
D)The major product under kinetic conditions is the 1,4 addition product because of proximity effects.
E)Neither carbocation stability nor proximity effects play a role in determining the preferred product under kinetic conditions.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
8
Which of these statements about thermodynamic and kinetic control is true?
A)The thermodynamic product is always different from the kinetic product.
B)Reactions under equilibrium conditions will favor the thermodynamic product.
C)A higher-energy transition state will favor the thermodynamic product.
D)The kinetic product will generally be favored at higher temperatures.
E)1,4-Addition gives the thermodynamic product because the intermediate cation is more stable.
A)The thermodynamic product is always different from the kinetic product.
B)Reactions under equilibrium conditions will favor the thermodynamic product.
C)A higher-energy transition state will favor the thermodynamic product.
D)The kinetic product will generally be favored at higher temperatures.
E)1,4-Addition gives the thermodynamic product because the intermediate cation is more stable.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
9
Which structure contains the longest C-C bond?
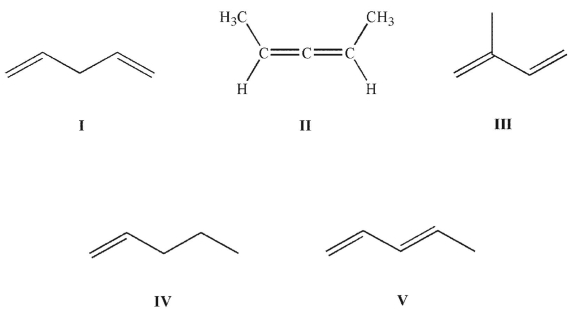
A)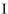
B)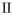
C)
D)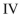
E)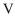

A)
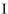
B)
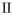
C)
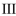
D)
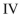
E)
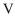

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the structures shown is in the s-cis conformation? 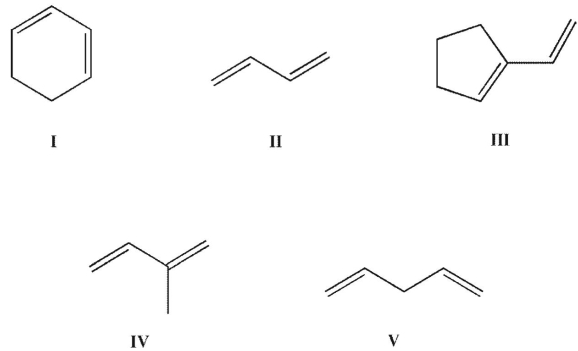
A)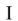
B)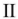
C)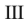
D)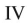
E)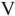

A)
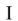
B)
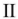
C)
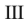
D)
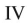
E)
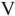

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
11
Rank these dienes in order of increasing heats of hydrogenation. 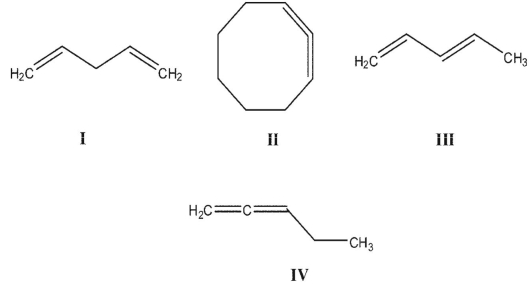
A)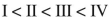
B)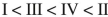
C)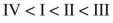
D)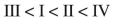
E)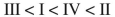

A)
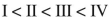
B)
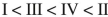
C)
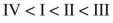
D)
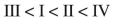
E)
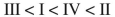

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the structures shown is in the s-trans conformation? 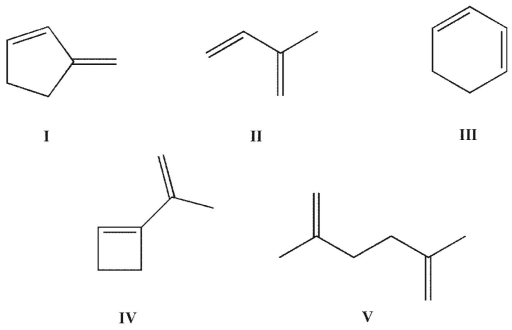
A)
B)
C)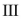
D)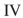
E)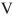

A)
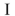
B)
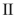
C)
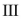
D)
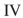
E)
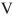

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following structures is the product of 1,2 addition of HCl to the molecule shown here? 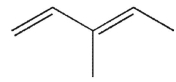
A) 4-chloro-2-methyl-1-hexene
B) 4-chloro-3-methyl-2-hexene
C) 3 -chloro-3-methyl-1-hexene
D) 5 -chloro-3-methyl-2-hexene
E) none of these

A) 4-chloro-2-methyl-1-hexene
B) 4-chloro-3-methyl-2-hexene
C) 3 -chloro-3-methyl-1-hexene
D) 5 -chloro-3-methyl-2-hexene
E) none of these

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
14
Which of these structures is a conjugated diene? 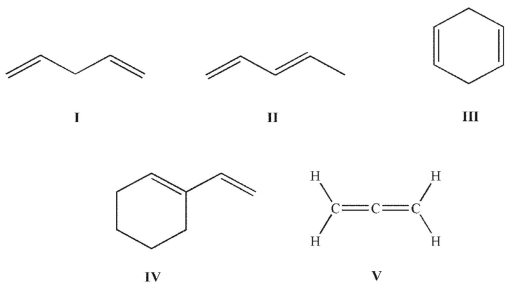
A) I and III
B) I, III, V
C) II and IV
D) II, IV, V
E) All of these structures are conjugated dienes

A) I and III
B) I, III, V
C) II and IV
D) II, IV, V
E) All of these structures are conjugated dienes

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
15
Which of these alkynes will not isomerize in the presence of NaNH2 ?
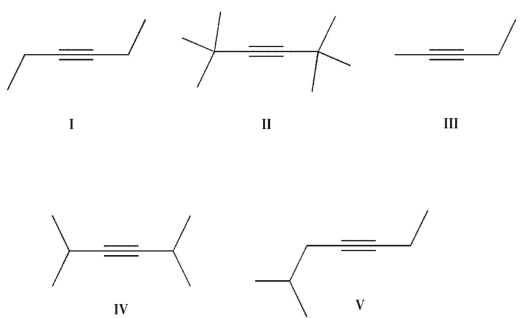
A) I
B) II
C) III and IV
D) II and IV
E) II, IV, and V

A) I
B) II
C) III and IV
D) II and IV
E) II, IV, and V

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
16
In the rearrangement of 2-butyne to 1-butyne, what causes the reaction to go to completion?
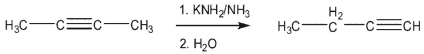
A) the increased stability of the terminal triple bond in the product
B) the high pKa of ammonia versus the methyl group in 2-butyne
C) the decreased activation energy for conversion of the allene intermediate to 1 -butyne
D) the much lower pKa of the acetylenic proton in the product versus the other species
E) the increased stability of the 1,2 -butadiene intermediate versus 2 -butyne

A) the increased stability of the terminal triple bond in the product
B) the high pKa of ammonia versus the methyl group in 2-butyne
C) the decreased activation energy for conversion of the allene intermediate to 1 -butyne
D) the much lower pKa of the acetylenic proton in the product versus the other species
E) the increased stability of the 1,2 -butadiene intermediate versus 2 -butyne

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
17
Which of these structures is the product of 1,4 addition of HBr to the molecule shown here? 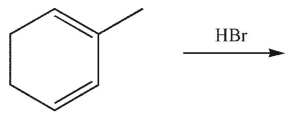
A)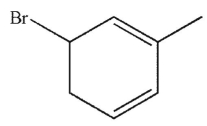
B)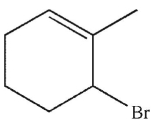
C)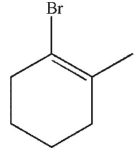
D)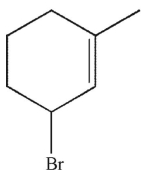
E)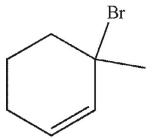

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
18
Which of these compounds can exist as cis/trans isomers?
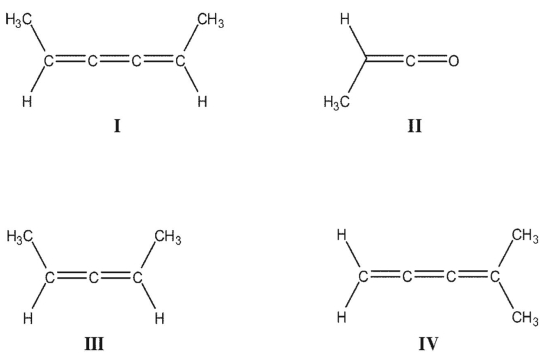
A) I
B) II
C) III
D) IV
E) none

A) I
B) II
C) III
D) IV
E) none

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
19
Rank the following C4H6 isomers in order of increasing ΔHf values:
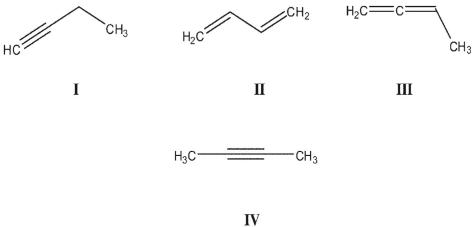
A)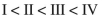
B)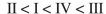
C)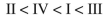
D)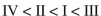
E)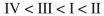

A)
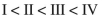
B)
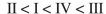
C)
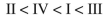
D)
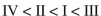
E)
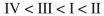

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following structures is chiral?
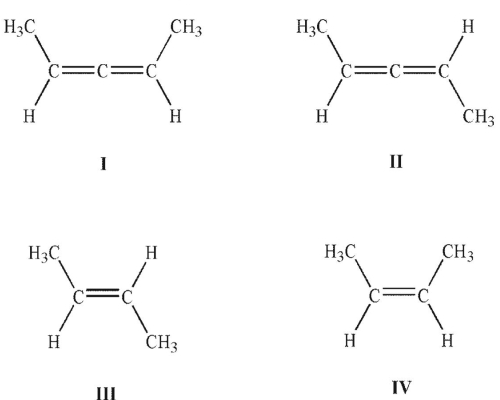
A) I
B) II
C) III
D) I and II
E) III and IV

A) I
B) II
C) III
D) I and II
E) III and IV

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
21
Draw a mechanism for the formation of the product resulting from 1,4 addition of HCl to the
molecule shown here.Include all lone pairs of electrons, curved arrows, and formal charges.
molecule shown here.Include all lone pairs of electrons, curved arrows, and formal charges.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
22
Draw the product of 1,2 addition of Br2 to the molecule shown here. 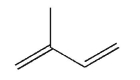


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following molecules could not be made using a Diels-Alder reaction?
A)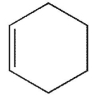
B)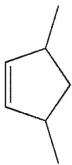
C)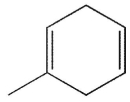
D)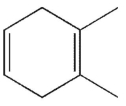
E)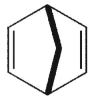
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following structures is the dienophile used to make the molecule shown here? 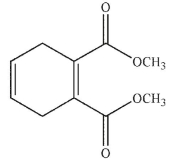
A)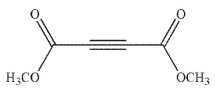
B)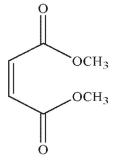
C)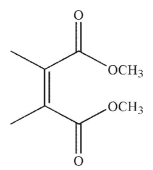
D)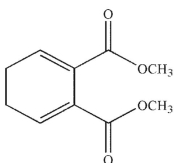
E)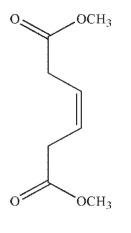

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following compounds could be classified as terpenes?
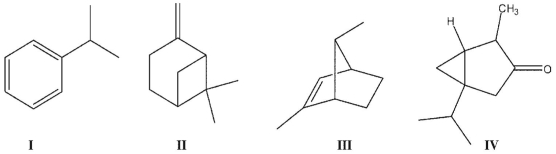
A) I and II
B) II and III
C) II and IV
D) III and IV
E) All can be classified as terpenes.

A) I and II
B) II and III
C) II and IV
D) III and IV
E) All can be classified as terpenes.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following structures is the product of the reaction shown here? 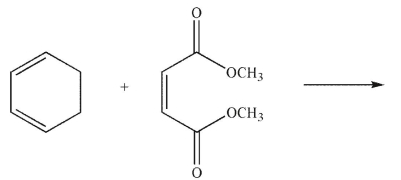
A)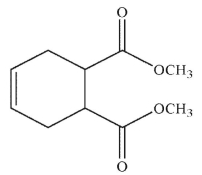
B)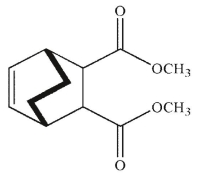
C)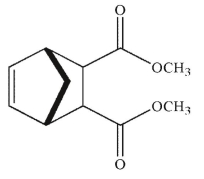
D)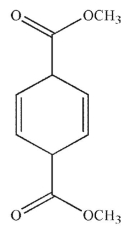
E)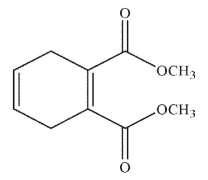

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following structures represents the basic steroid ring system?
A)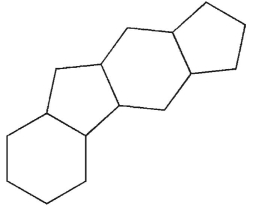
B)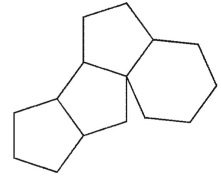
C)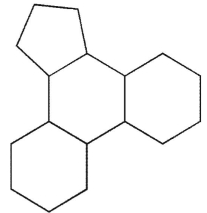
D)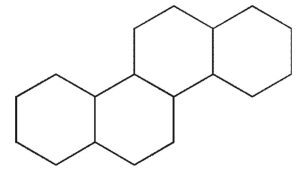
E)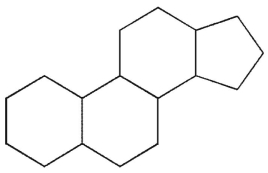
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
28
Draw the product of 1,4 addition of Cl2 to the molecule shown here. 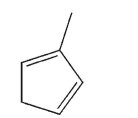


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
29
Draw a mechanism for the isomerization of the starting material to the product shown using the reagents given.
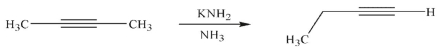


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
30
Rate the following three C7H12 isomers from lowest to highest enthalpy of hydrogenation.
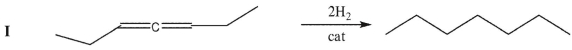
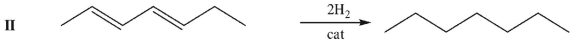
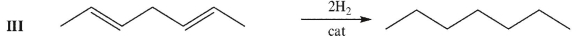
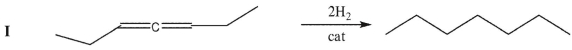
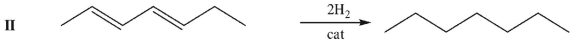
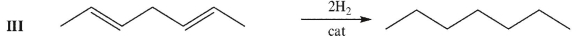

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
31
Draw a mechanism for the formation of the product resulting from 1,2 addition of HBr to the
molecule shown here.Include all lone pairs of electrons, curved arrows, and formal charges.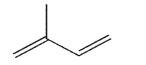
molecule shown here.Include all lone pairs of electrons, curved arrows, and formal charges.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
32
Rank the following  isomers in order of increasing heats of formation.
isomers in order of increasing heats of formation.
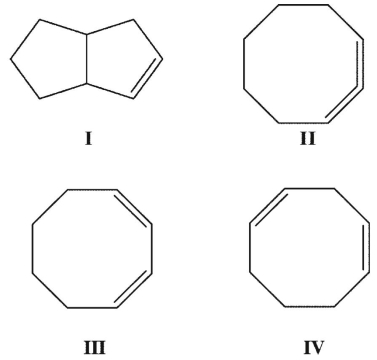
 isomers in order of increasing heats of formation.
isomers in order of increasing heats of formation.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following dienes could react with acrylonitrile in a Diels-Alder reaction? 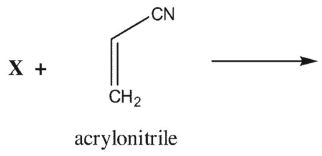
A)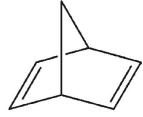
B)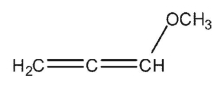
C)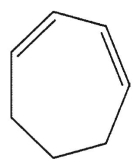
D)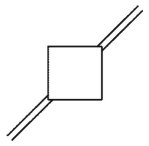
E)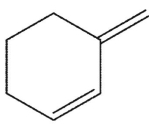

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
34
Which of these structures is isoprene?
A)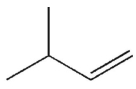
B)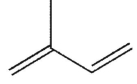
C)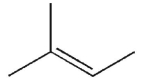
D)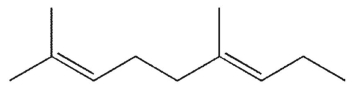
E)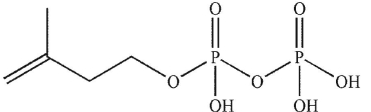
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
35
Which pairing of diene and dienophile would lead to the product shown here? 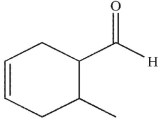
A)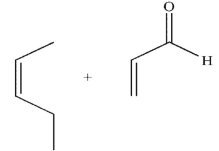
B)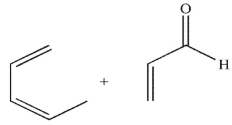
C)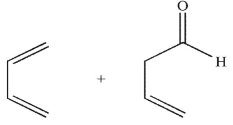
D)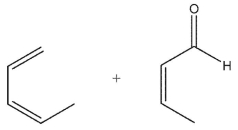
E)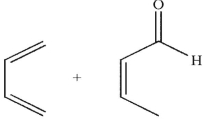

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following compounds gives only one constitutional isomer when it undergoes solvolysis in water? (You may ignore stereochemistry for the purposes of this question.)
A)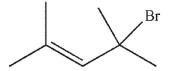
B)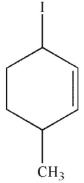
C)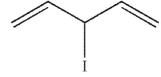
D)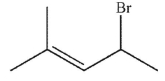
E)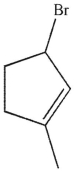
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
37
Which of these orbitals is the HOMO in the allyl anion?
A)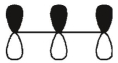
B)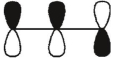
C)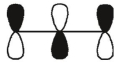
D)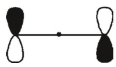
E)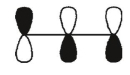
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following statements about the Diels-Alder reaction is true?
A)The endo product is kinetically favored.
B)The exo product is kinetically favored.
C)The endo product is more stable.
D)The exo product is more stable.
E)both a and d
A)The endo product is kinetically favored.
B)The exo product is kinetically favored.
C)The endo product is more stable.
D)The exo product is more stable.
E)both a and d

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following words best describes the major product(s) of the following reaction?
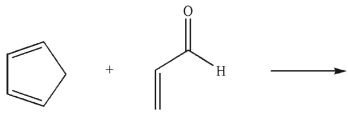
A) structural isomers
B) a single compound
C) racemic mixture
D) diastereomers
E) an unequal mixture of enantiomers

A) structural isomers
B) a single compound
C) racemic mixture
D) diastereomers
E) an unequal mixture of enantiomers

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following do you expect to react fastest in an 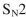 reaction with cyanide ion?
reaction with cyanide ion?
A)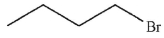
B)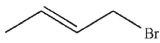
C)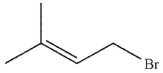
D)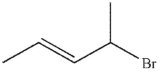
E)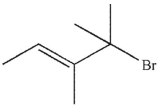
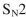 reaction with cyanide ion?
reaction with cyanide ion?A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
41
Which molecular orbitals are involved in each reactant in the Diels-Alder reaction?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
42
Draw the lettered intermediates and products in the following synthetic sequence.Indicate the correct stereochemistry as needed. 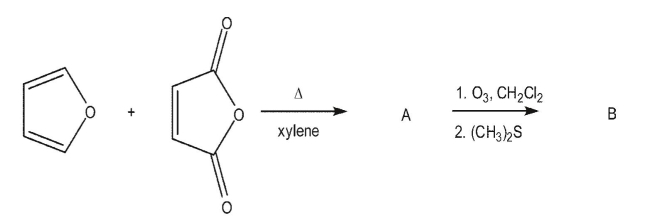


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
43
Provide the structures of the diene and the dienophile that were combined in a Diels-Alder reaction to form the structure shown. 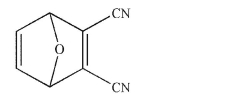


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
44
Draw the endo product of the Diels-Alder reaction shown here. 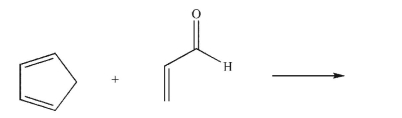


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
45
Draw the structures of the diene and dienophile used to synthesize the product shown here. 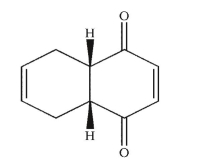


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
46
For each pair of reactions, indicate which would proceed at the faster rate. 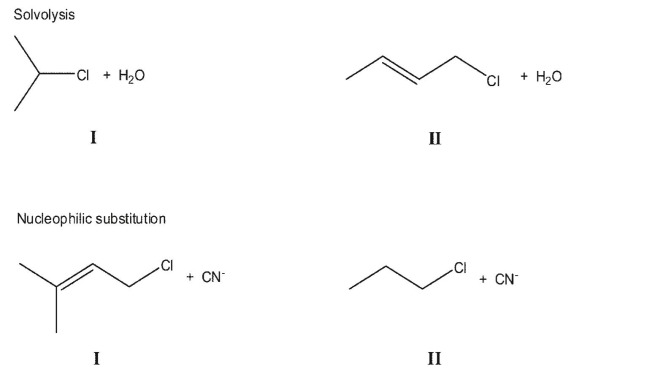


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
47
Draw the major product in the following reaction. 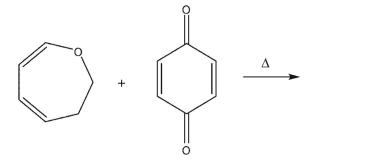


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
48
Draw the product of the reaction shown here. 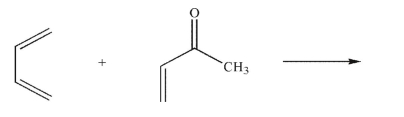


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
49
Draw the products of the addition reaction under each of the conditions shown; indicate the major and minor product isomers in each case: 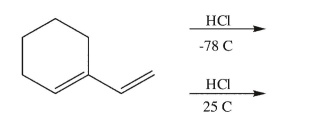


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
50
Draw a mechanism for the reverse Diels-Alder reaction that occurs when the following molecule
is heated.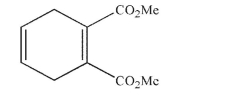
is heated.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
51
Draw the kinetic and thermodynamic products of the following reaction.Indicate the general
conditions required to form each.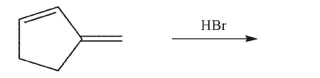
conditions required to form each.
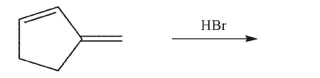

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
52
Draw the structure of the product formed when cyclopentadiene dimerizes in a Diels-Alder
reaction.
reaction.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
53
Draw a mechanism for the transformation shown here.Include all lone pairs of electrons, curved arrows, and formal charges. 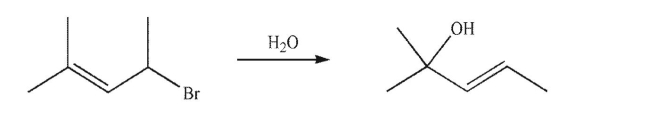


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
54
Draw the exo product of the Diels-Alder reaction shown here. 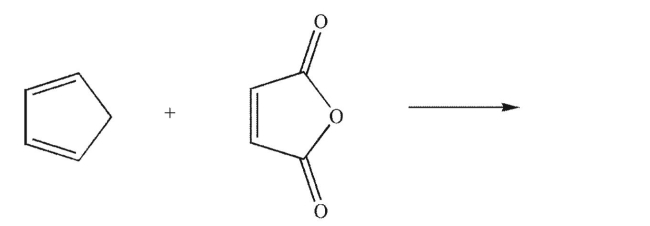


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
55
Predict the product of the following reaction and draw an arrow-pushing mechanism for its formation.Include all lone pairs, curved arrows, and formal charges. 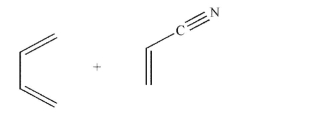


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
56
Estimate the enthalpy change for the reaction shown here using the bond dissociation energies
given.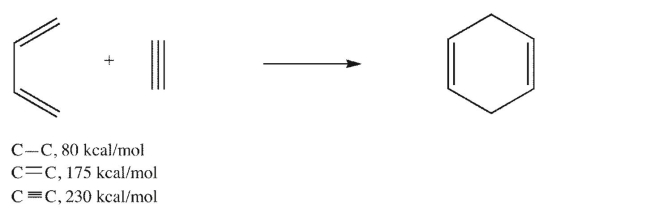
given.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
57
Draw a mechanism for the transformation shown here.Include all lone pairs of electrons, curved arrows, and formal charges. 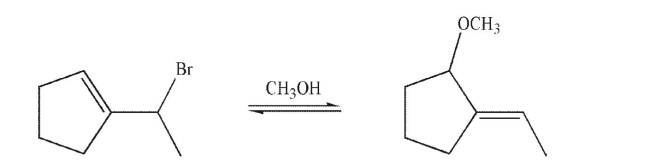


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
58
Devise a multistep synthesis for the following transformation.You may use any organic or inorganic reagents of your choice.Include the reagents necessary for each step and the product of each step. 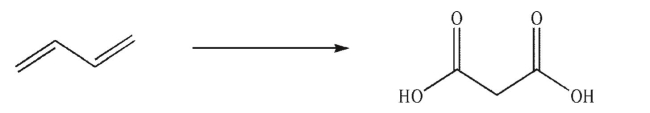


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
59
SN1 solvolysis of each of the two bromoalkenes shown gives the same mixture of two alcohols. Draw the two alcohol products and indicate which would be the major and minor isomers.
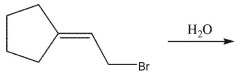




Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
60
Explain the difference between the two following molecules in their SN2 reactivity with hydroxide ion.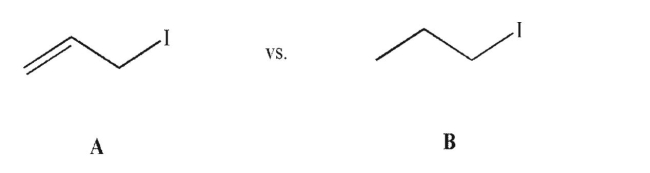


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
61
Draw the structures of the diene and dienophile used to synthesize the molecule shown here using a
Diels-Alder reaction.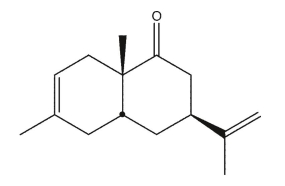
Diels-Alder reaction.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
62
Identify the isoprene units in this structure. 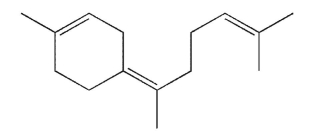


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
63
Devise a multistep synthesis for the following transformation.You may use any organic or inorganic reagents of your choice.Include the reagents necessary for each step and the product of each step. 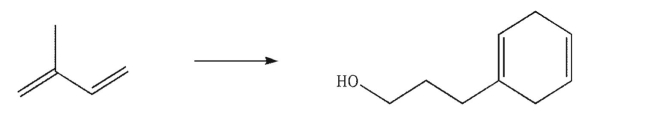


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
64
Convert the structure shown here to a bond-line formula showing appropriate stereochemistry at
all asymmetric carbons.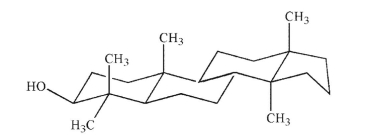
all asymmetric carbons.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
65
Devise a multistep synthesis for the following transformation.You may use any organic or inorganic reagents of your choice.Include the reagents necessary for each step and the product of each step. 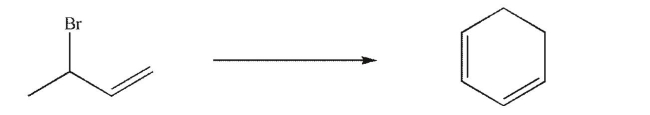


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
66
The molecule shown here can undergo an intramolecular Diels-Alder reaction.Draw the product of this reaction. 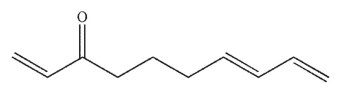


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
67
Identify the isoprene units in this structure. 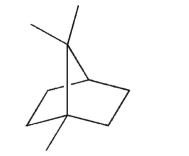


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
68
Draw the products of the reaction shown here, including stereochemistry. 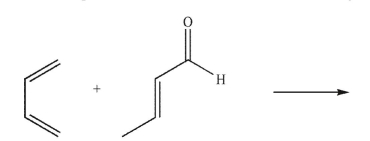


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck


