Deck 11: More Additions to Bonds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/65
Play
Full screen (f)
Deck 11: More Additions to Bonds
1
Which of the following is the major product of this reaction?
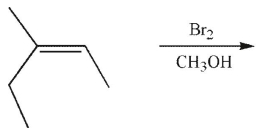
A)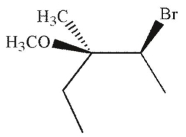
B)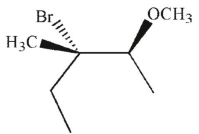
C)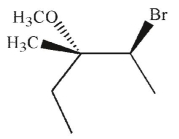
D)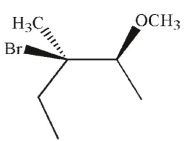
E)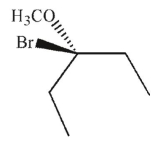

A)

B)

C)

D)

E)


2
Which of the following statements about the reaction shown here is false?
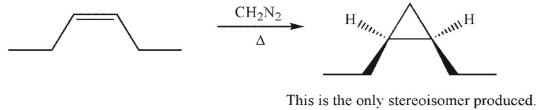
A) The reaction is a stereospecific syn addition.
B) The reactive intermediate involved in this process is a triplet carbene.
C) The mechanism is concerted.
D) Nitrogen gas is produced in the reaction.
E) The reactive intermediate in this process is a neutral species.

A) The reaction is a stereospecific syn addition.
B) The reactive intermediate involved in this process is a triplet carbene.
C) The mechanism is concerted.
D) Nitrogen gas is produced in the reaction.
E) The reactive intermediate in this process is a neutral species.
The reactive intermediate involved in this process is a triplet carbene.
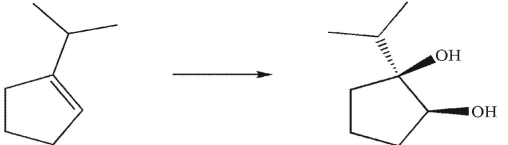
3
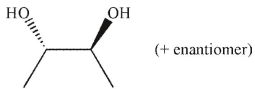
What was the starting material used to produce the diol shown under the conditions given?
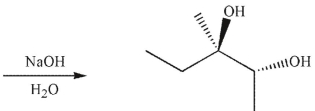
A)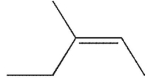
B)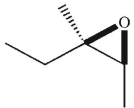
C)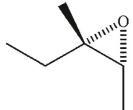
D)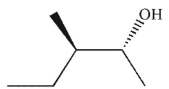
E)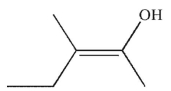

A)

B)

C)

D)

E)


4
Which product will be observed?
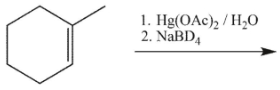
A)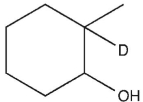
B)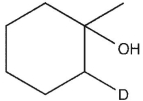
C)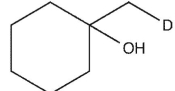
D)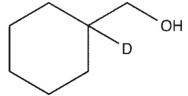
E)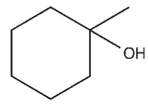
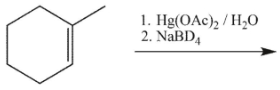
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
5
Which of these molecules is the product of the reaction shown here?
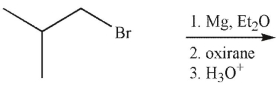
A)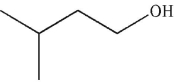
B)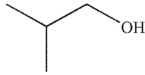
C)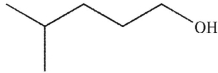
D)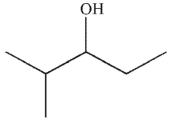
E)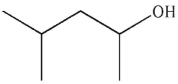

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
6
which product will be observed?
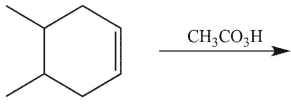
A)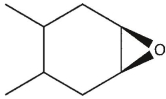
B)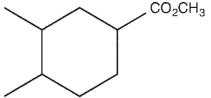
C)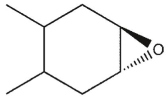
D)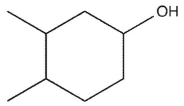
E)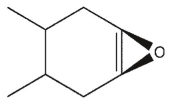

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
7
Which product(s) will be observed?
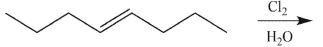
A)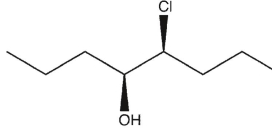
B)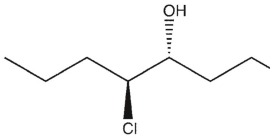
C) Both a and b
D)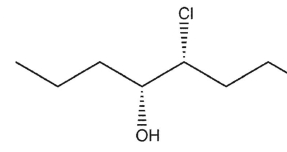
E)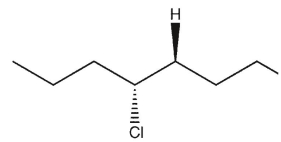

A)

B)

C) Both a and b
D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
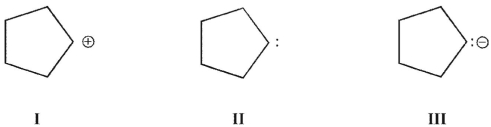
8
Which of these structures is a carbene?
A) I
B) II
C) III
D) II and III
E) I, II, and III

A) I
B) II
C) III
D) II and III
E) I, II, and III

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
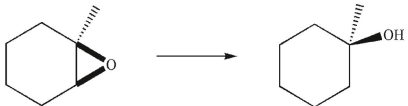
9
Which reagent would you use to accomplish the transformation shown here?
A)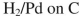
B)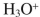
C)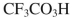
D)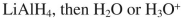
E)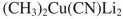

A)
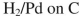
B)
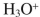
C)
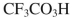
D)
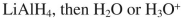
E)
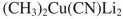

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
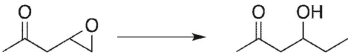
10
Select the best reagent to accomplish the transformation shown here.
A)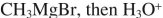
B)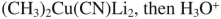
C)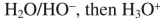
D)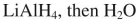
E)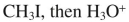

A)
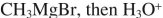
B)
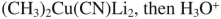
C)
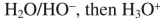
D)
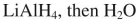
E)
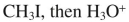

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
11
Which product(s) will be observed?
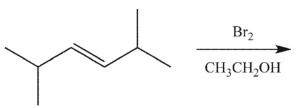
A)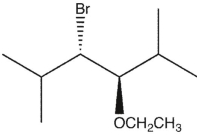
B)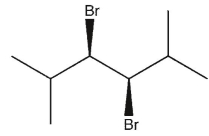
C) Both a and d
D)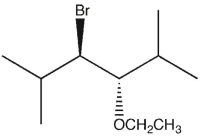
E)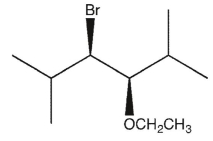

A)

B)

C) Both a and d
D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
12
Which product(s) will be observed?
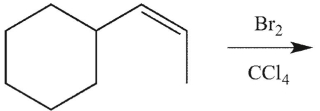
A)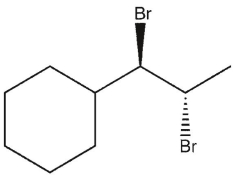
B)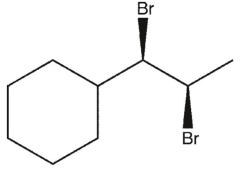
C)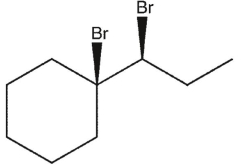
D)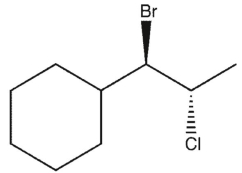
E) Both a and b

A)

B)

C)

D)

E) Both a and b

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
13
Which of these structures is an intermediate in this transformation?
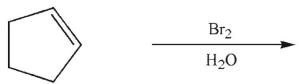
A)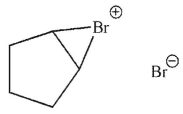
B)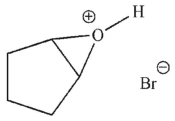
C)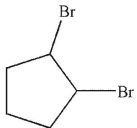
D)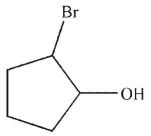
E)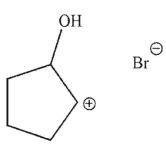
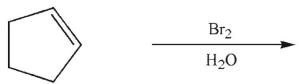
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
14
Which reagent would you use to accomplish the transformation shown here?

A)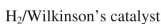
B)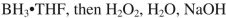
C)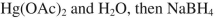
D)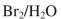
E)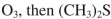

A)
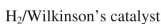
B)
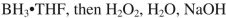
C)
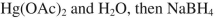
D)
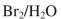
E)
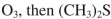

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
15
Which product will be obsereced?
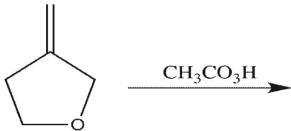
A)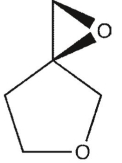
B)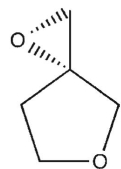
C)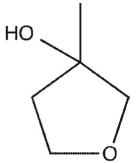
D)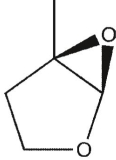
E) Boh a and b

A)

B)

C)

D)

E) Boh a and b

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
16
Consider alkenes A and B .
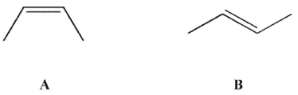
Which of the following is easily accessible from either A or B ?
A)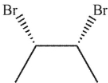
B)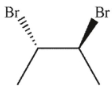
C)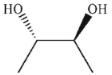
D)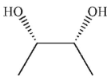
E) Both c and d
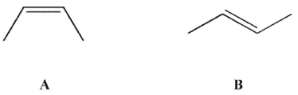
Which of the following is easily accessible from either A or B ?
A)

B)

C)

D)

E) Both c and d

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
17
Which reagent would you use to accomplish the transformation shown here?

A) Br2/H2O
B) HO-/ H2O
C) H3O+/ H2O
D) Hg(OAc)2 and H2O, then NaBH4
E) CF3CO3H

A) Br2/H2O
B) HO-/ H2O
C) H3O+/ H2O
D) Hg(OAc)2 and H2O, then NaBH4
E) CF3CO3H

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
18
Which reagent would you use to accomplish the transformation shown here?
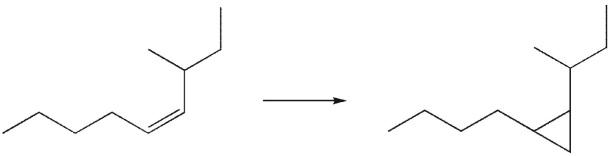
A) diazomethane
B) bromoform, then potassium tert-butoxide
C) trifluoroperacetic acid
D) phenylmagnesium bromide in ether, followed by aqueous acid workup
E) osmium tetroxide

A) diazomethane
B) bromoform, then potassium tert-butoxide
C) trifluoroperacetic acid
D) phenylmagnesium bromide in ether, followed by aqueous acid workup
E) osmium tetroxide

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
19
Which of these molecules is the product of the reaction shown here?
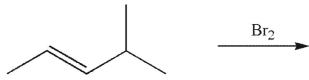
A)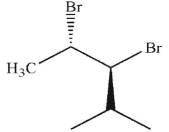
B)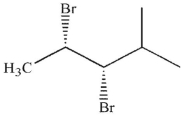
C)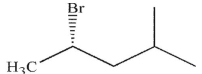
D)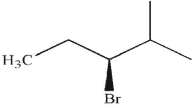
E)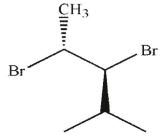

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
20
Which product will be observed?
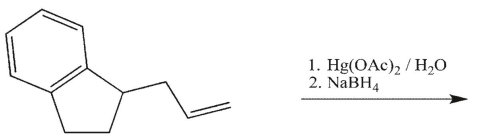
A)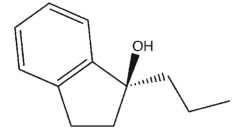
B)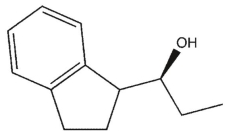
C)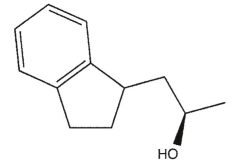
D)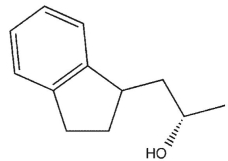
E) c and d

A)

B)

C)

D)

E) c and d

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
21
Draw the products of the following reaction.Show stereochemistry and indicate the stereochemical
relationship of the products to each other.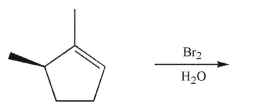
relationship of the products to each other.


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following conditions and reagents were used to make this compound?
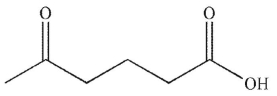
A)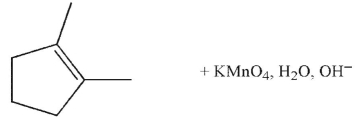
B)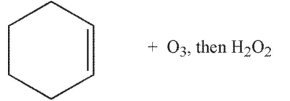
C)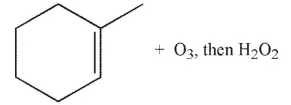
D)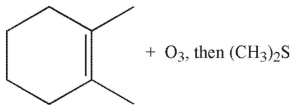
E)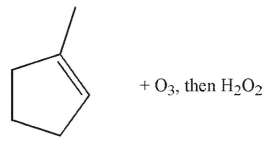

A)
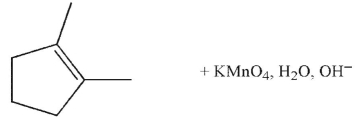
B)
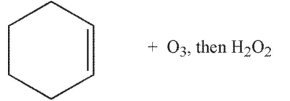
C)

D)
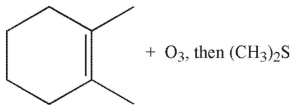
E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following structures is an enol?
A)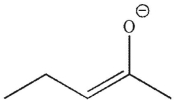
B)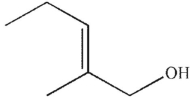
C)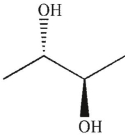
D)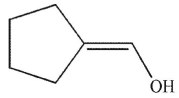
E)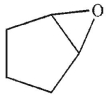
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
24
Predict the product and draw a mechanism for its formation in the following reaction.Show all lone pairs, curved arrows, and nonzero formal charges. 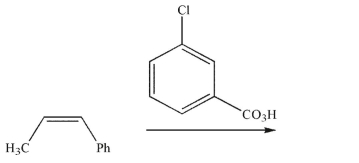


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
25
Which enol, when treated with aqueous acid or base, would generate the ketone shown here? 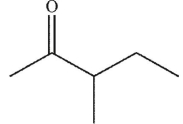
A)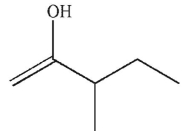
B)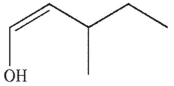
C)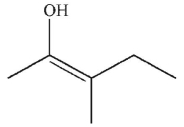
D)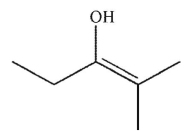
E) Both a and c

A)

B)

C)

D)

E) Both a and c

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
26
Which reagent would you use to accomplish the following transformation?
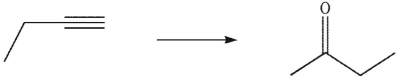
A)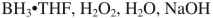
B)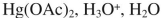
C)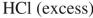
D)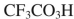
E)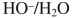

A)
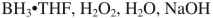
B)
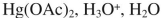
C)
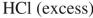
D)
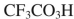
E)
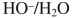

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following statements about treatment of an alkyne with sodium metal in ammonia is false? 
A) The product of the reaction is a trans alkene.
B) A radical anion is protonated by solvent.
C) This reaction provides the opposite stereochemistry to that of treating an alkyne with H2 in the presence of Lindlar's catalyst.
D) Sodium amide forms as the reaction proceeds.
E) A single electron is donated by sodium to a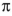 orbital in the alkyne.
orbital in the alkyne.

A) The product of the reaction is a trans alkene.
B) A radical anion is protonated by solvent.
C) This reaction provides the opposite stereochemistry to that of treating an alkyne with H2 in the presence of Lindlar's catalyst.
D) Sodium amide forms as the reaction proceeds.
E) A single electron is donated by sodium to a
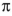 orbital in the alkyne.
orbital in the alkyne.
Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
28
Which of these products would result from complete ozonolysis of (S)-(+) -carvone, followed by oxidative workup? The structure of racemic carvone is shown.
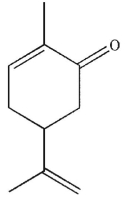
A)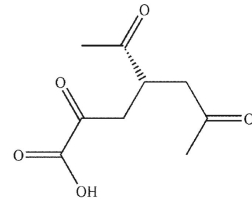
B)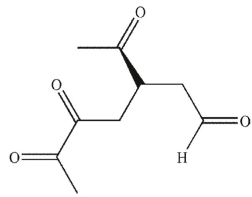
C)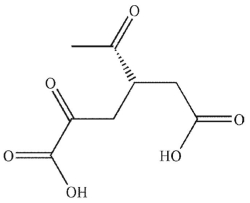
D)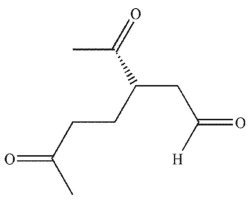
E)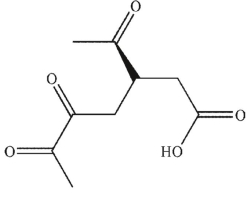

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
29
Provide an arrow-pushing mechanism for the formation of the product shown.Your mechanism
should include stereochemistry and should result in the specific stereoisomer shown as the
product.Include all lone pairs, curved arrows, and nonzero formal charges.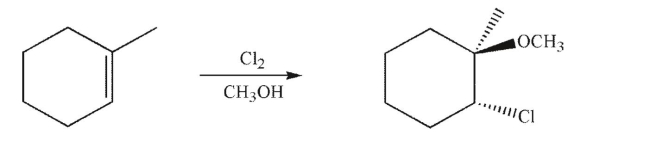
should include stereochemistry and should result in the specific stereoisomer shown as the
product.Include all lone pairs, curved arrows, and nonzero formal charges.


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following molecules do you expect to react fastest with H2(g) and palladium on charcoal?
A)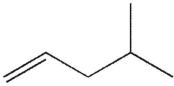
B)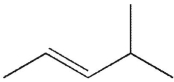
C)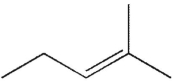
D)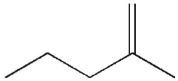
E)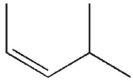
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
31
Starting with an alkene, which of these methods would produce at least one carbonyl compound as the major product? Assume appropriate aqueous workup in all cases.
A) OsO4
B) H3O+
C) H2 and Lindlar's catalyst
D) O3, then H2O2
E) Hg(OAc)2 and H2O , then NaBH4
A) OsO4
B) H3O+
C) H2 and Lindlar's catalyst
D) O3, then H2O2
E) Hg(OAc)2 and H2O , then NaBH4

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
32
Which reagent would you use to accomplish the following transformation? 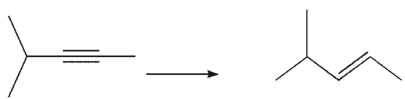
A) H2/ Pd on C.
B) H2/Wilkinson's catalyst
C) CF3CO3H
D) Na/ NH3
E) H2/ Lindlar's catalyst

A) H2/ Pd on C.
B) H2/Wilkinson's catalyst
C) CF3CO3H
D) Na/ NH3
E) H2/ Lindlar's catalyst

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
33
Draw the products of the following reaction.Show stereochemistry and indicate the
stereochemical relationship of the products to each other.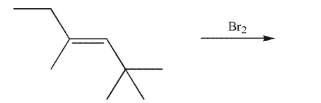
stereochemical relationship of the products to each other.


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
34
In the presence of aqueous acid or base, enols are converted to ketones or aldehydes.Which of the following carbonyl compounds is produced when the enol shown here is treated with aqueous
Acid?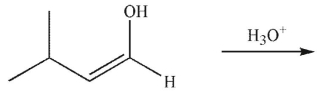
A)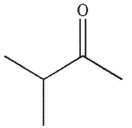
B)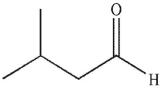
C)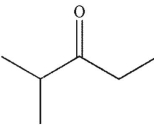
D)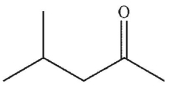
E)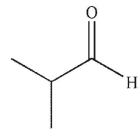
Acid?

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
35
Which of these enols could be generated by treating an alkyne with the reagent shown here, followed by treatment with aqueous, basic hydrogen peroxide?
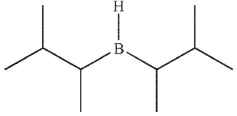
A)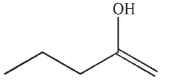
B)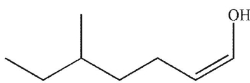
C)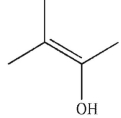
D)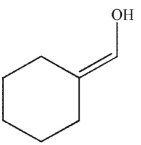
E) Both a and b

A)

B)

C)

D)

E) Both a and b

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
36
How would you make this compound in one step from alkene of formula C4H8 ?
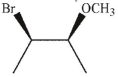


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is not a 1,3-dipolar reagent?
A)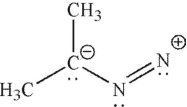
B)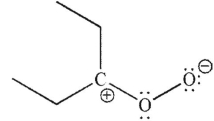
C)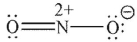
D)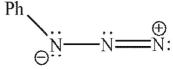
E)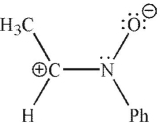
A)

B)

C)
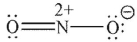
D)
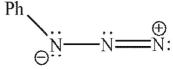
E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
38
Which reagent would you use to accomplish the transformation shown here?
A)
B)
C)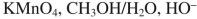
D)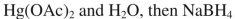
E)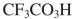

A)
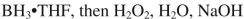
B)
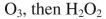
C)
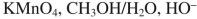
D)
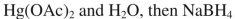
E)
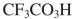

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following conditions will result in the formation of the molecule shown here?
A)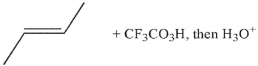
B)
C)
D)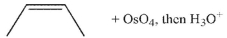
E) Both b and c will work.

A)
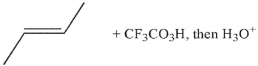
B)
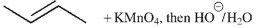
C)
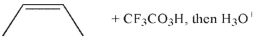
D)
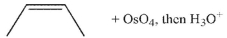
E) Both b and c will work.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
40
Which of these products would result from complete ozonolysis of (R) - (+) -limonene, followed by reductive workup? The structure of racemic limonene is shown.
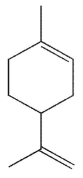
A)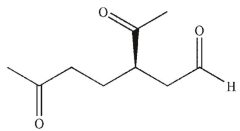
B)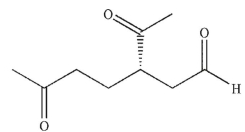
C)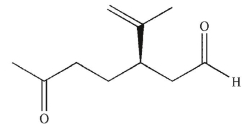
D)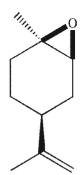
E)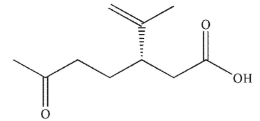

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
41
List three ways to accomplish the transformation shown here. 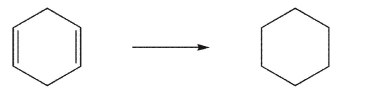


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
42
Draw a mechanism for the transformation shown.Include all curved arrows, lone pairs of
electrons, and nonzero formal charges.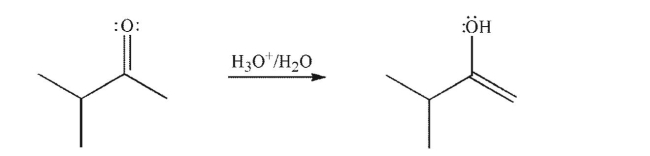
electrons, and nonzero formal charges.


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
43
Show how you would make each of the carbonyl compounds shown using the starting material
provided and any reagents of your choice.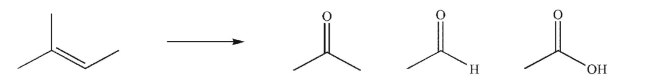
provided and any reagents of your choice.


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
44
When the diene shown here is treated with hydrogen and palladium on charcoal for a short reaction time, the alkene on the left is produced. When the same diene is treated with CF3CO3H for a short reaction time, the epoxide on the right is produced. Explain the difference in reactivity between the two alkenes.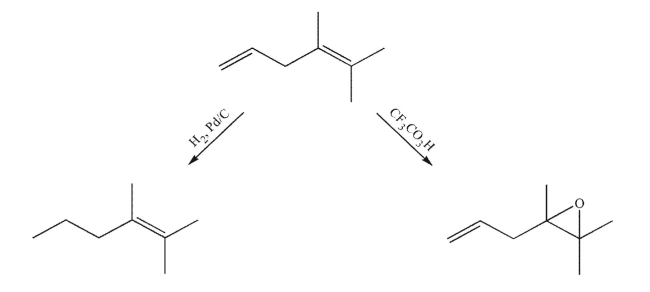


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
45
Propose a mechanism for the transformation shown here. 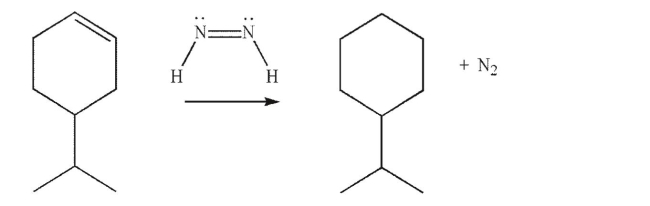


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
46
Draw the structure of the most stable ozonide that will result when the compound shown here is
treated with ozone.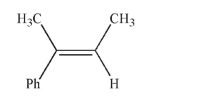
treated with ozone.


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
47
Predict the product of the following transformation, including the correct stereochemistry. 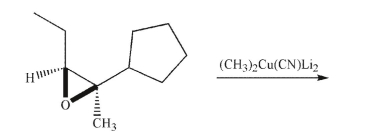


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
48
Draw the product of the following transformation: 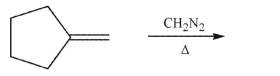
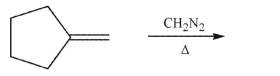

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
49
Draw a mechanism for the following transformation.Show all lone pairs, curved arrows, and
nonzero formal charges.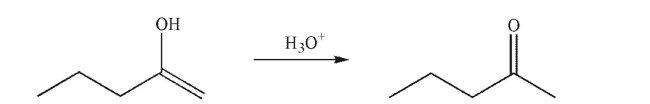
nonzero formal charges.


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
50
Devise a multistep synthesis of the target molecule from the given starting material.Show the
reagents needed for each step and the product of each step.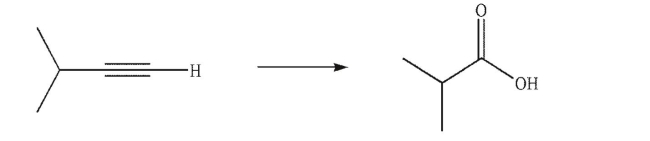
reagents needed for each step and the product of each step.


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
51
The following transformation was carried out using one enantiomer of diethyl tartrate and the other reagents of the Sharpless asymmetric epoxidation reaction.
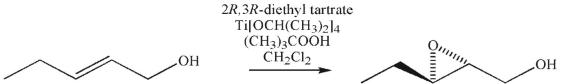
Predict the product that would result using the other enantiomer of diethyl tartrate.

Predict the product that would result using the other enantiomer of diethyl tartrate.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
52
The molecule shown was the exclusive product of an ozonolysis reaction followed by a reductive
workup.Draw the structures of two possible starting materials that could have been used.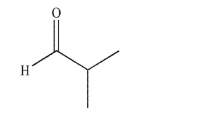
workup.Draw the structures of two possible starting materials that could have been used.


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
53
Draw the structure of the enol intermediate that would result from the following conditions. 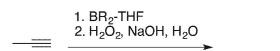


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
54
Provide two different precursors and the reagents needed to transform them to the compound shown
here.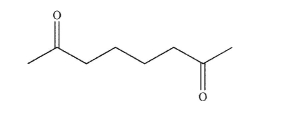
here.


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
55
Provide the missing reagents and structures.
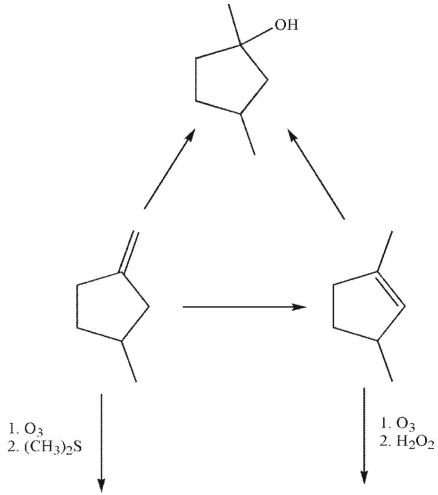


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
56
Explain the difference between a heterogeneous catalyst and a homogeneous catalyst.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
57
Provide the missing reagents in the following reaction sequence. 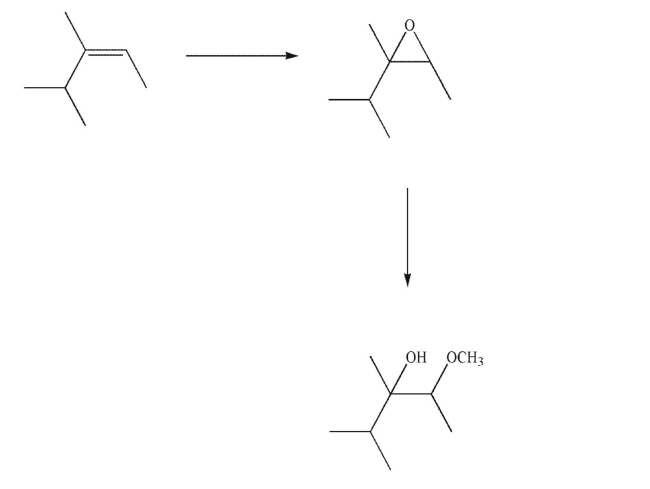


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
58
Predict the product of the following transformation. 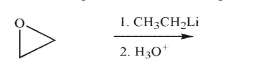
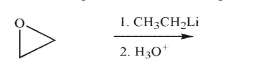

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
59
Explain the difference between a triplet carbene and a singlet carbene.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
60
Propose three different syntheses of the specific enantiomer of the diol shown here.Each
synthesis must involve a different starting material.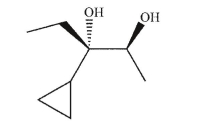
synthesis must involve a different starting material.


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
61
Devise a multistep synthesis of the target molecule from the given starting material. Show the reagents needed for each step and the product of each step.
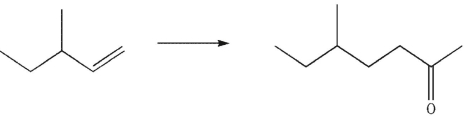


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
62
Devise a multistep synthesis of the target molecule from the given starting material.Show the
reagents needed for each step and the product of each step.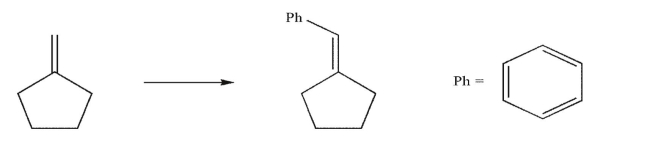
reagents needed for each step and the product of each step.


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
63
Devise a multistep synthesis of the target molecule from the given starting material.Show the
reagents needed for each step and the product of each step.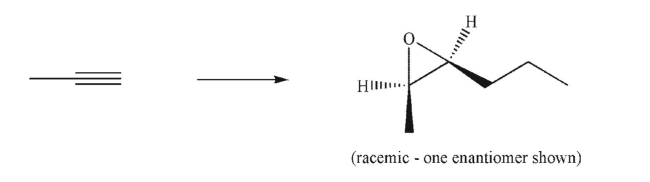
reagents needed for each step and the product of each step.


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
64
Devise a multistep synthesis of the target molecule from the given starting material.Show the
reagents needed for each step and the product of each step.Assume that the target is racemic; only
one enantiomer is shown.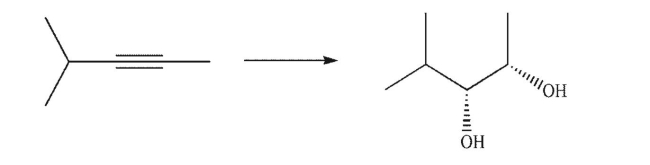
reagents needed for each step and the product of each step.Assume that the target is racemic; only
one enantiomer is shown.


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
65
Devise a multistep synthesis of the target molecule from the given starting material.Show the
reagents needed for each step and the product of each step.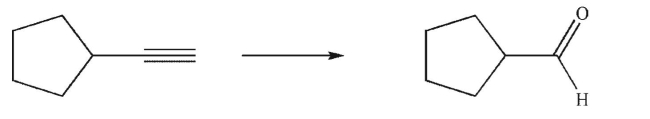
reagents needed for each step and the product of each step.


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck


