Deck 15: Substitution Reactions of Aromatic Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/68
Play
Full screen (f)
Deck 15: Substitution Reactions of Aromatic Compounds
1
Why is one equivalent of AlCl3 required for the transformation shown here?

A) Either the oxygen or the chlorine in the acyl chloride can attach to the Al atom in AlCl3.
B) AlCl3 is needed to produce the acyl chloride during the course of the reaction.
C) The product forms a complex with the AlCl3.
D) The product would rearrange unless there were extra. AlCl3 present.
E) Both benzene and the acyl chloride form complexes with the AlCl3.

A) Either the oxygen or the chlorine in the acyl chloride can attach to the Al atom in AlCl3.
B) AlCl3 is needed to produce the acyl chloride during the course of the reaction.
C) The product forms a complex with the AlCl3.
D) The product would rearrange unless there were extra. AlCl3 present.
E) Both benzene and the acyl chloride form complexes with the AlCl3.
The product forms a complex with the AlCl3.
2
Which reagent would you use to accomplish the transformation shown?

A) Br2
B) Br2/FeBr3
C)CuBr
D)Br2/AlBr3
E) HBr

A) Br2
B) Br2/FeBr3
C)CuBr
D)Br2/AlBr3
E) HBr
CuBr
3
Which conditions would you use to accomplish the following transformation?

A) HNO3/H2SO4
B)KOH, H2NNH2, ethylene glycol
C)HNO3/ H2SO4, then H2/Pd/C
D)HNO3/H2SO4, then H2O
E) Na, NH3

A) HNO3/H2SO4
B)KOH, H2NNH2, ethylene glycol
C)HNO3/ H2SO4, then H2/Pd/C
D)HNO3/H2SO4, then H2O
E) Na, NH3
HNO3/ H2SO4, then H2/Pd/C
4
What is the electrophile in this reaction? 
A) HNO3
B) H2SO4
C) SO3
D) NO2+
E) Benzene

A) HNO3
B) H2SO4
C) SO3
D) NO2+
E) Benzene

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements is false?
A)Hydrogenation of benzene is possible.
B)Hydrogenation of benzene is endothermic.
C)Hydrogenation of benzene requires special conditions, such as increased heat or pressure or special catalysts.
D)Hydrogenation of benzene requires 3 moles of hydrogen gas.
E)The transformation of benzene to cyclohexane has a high activation energy.
A)Hydrogenation of benzene is possible.
B)Hydrogenation of benzene is endothermic.
C)Hydrogenation of benzene requires special conditions, such as increased heat or pressure or special catalysts.
D)Hydrogenation of benzene requires 3 moles of hydrogen gas.
E)The transformation of benzene to cyclohexane has a high activation energy.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
6
What is the proper description of the compound shown here?

A) diazonium salt
B) nitroso compound
C) aniline
D) anilinium ion
E) diazotic acid

A) diazonium salt
B) nitroso compound
C) aniline
D) anilinium ion
E) diazotic acid

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
7
What is the electrophile in this reaction?

A) Br2
B) FeBr3
C) FeBr4-
D) Benzene
E)FeBr5+

A) Br2
B) FeBr3
C) FeBr4-
D) Benzene
E)FeBr5+

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following compounds is activated in electrophilic aromatic substitution reaction relative to benzene?

A) I
B) I and III
C) III
D) IV
E) II and IV

A) I
B) I and III
C) III
D) IV
E) II and IV

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following would not react with the compound shown?

A) KI
B) CuCN
C) CuBr
D) HCl
E) HBF4

A) KI
B) CuCN
C) CuBr
D) HCl
E) HBF4

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
10
What is the electrophile in this reaction?

A) H2SO4
B) SO3
C) HSO3+
D) Benzene
E) HSO4-

A) H2SO4
B) SO3
C) HSO3+
D) Benzene
E) HSO4-

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements is true?
A)All meta directors are electron-withdrawing groups.
B)All electron-withdrawing groups are meta directors.
C)All ortho, para directors are activating.
D)All ortho, para directors are deactivating.
E)All electron-donating groups are meta directors.
A)All meta directors are electron-withdrawing groups.
B)All electron-withdrawing groups are meta directors.
C)All ortho, para directors are activating.
D)All ortho, para directors are deactivating.
E)All electron-donating groups are meta directors.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following substrates would not provide a rearranged product when reacted with benzene in a Friedel-Crafts alkylation reaction?
A)
B)
C)
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
13
What is the electrophile in the reaction shown here?

A)
B) AlCl3
C)
D)
E) Benzene

A)

B) AlCl3
C)

D)

E) Benzene

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following statements is false?
A)Halogenated benzenes have higher boiling points than benzene.
B)Halogen atoms donate electron density to the ring by resonance.
C)Halogen atoms are deactivating in electrophilic aromatic substitution.
D)Halogen atoms are meta directors.
E)Halogen atoms withdraw electron density from the ring inductively.
A)Halogenated benzenes have higher boiling points than benzene.
B)Halogen atoms donate electron density to the ring by resonance.
C)Halogen atoms are deactivating in electrophilic aromatic substitution.
D)Halogen atoms are meta directors.
E)Halogen atoms withdraw electron density from the ring inductively.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
15
Which of these compounds is the product of the reaction shown? 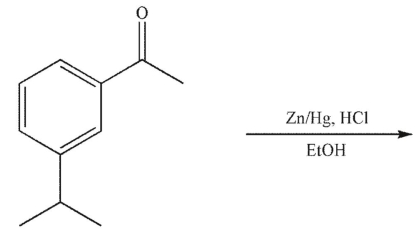
A)

B)

C)

D)

E)


A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
16
What is the product of the following reaction?

A)

B)

C)

D)

E)
No reaction occurs.

A)

B)

C)

D)

E)
No reaction occurs.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
17
What is the missing reagent in the following reaction sequence?

A) CO, HCl
B) H3O+
C) NaNO2 , then H3PO2
D) H3PO2
E) Na, NH3

A) CO, HCl
B) H3O+
C) NaNO2 , then H3PO2
D) H3PO2
E) Na, NH3

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following compounds is deactivated in electrophilic aromatic substitution reaction relative to benzene?

A) I
B) I and II
C) III
D) IV
E) IV and V

A) I
B) I and II
C) III
D) IV
E) IV and V

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
19
What is the major product of nitration of thiophene?

A)

B)

C)

D)

E) No reaction occurs.

A)

B)

C)

D)

E) No reaction occurs.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
20
Which reagent set could not be used to accomplish the transformation shown here?

A) H2/ Pd / C, EtOH
B) Sn/HCl , then OH-(aq)
C) LiAlH4, then H2O
D) KMnO4
E) any of these reagents could be used

A) H2/ Pd / C, EtOH
B) Sn/HCl , then OH-(aq)
C) LiAlH4, then H2O
D) KMnO4
E) any of these reagents could be used

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following statements about benzyne is false?
A) The geometry around the alkyne is bent.
B) There are four hydrogen atoms.
C) Each of the two π because bonds in the alkyne results from overlap of 2p orbitals on adjacent carbons.
D) One of the π bonds is involved in the ring's π system, while the other is not.
E) The compound is strained and highly reactive.
A) The geometry around the alkyne is bent.
B) There are four hydrogen atoms.
C) Each of the two π because bonds in the alkyne results from overlap of 2p orbitals on adjacent carbons.
D) One of the π bonds is involved in the ring's π system, while the other is not.
E) The compound is strained and highly reactive.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
22
Which of these is the product of the reaction shown here? 
A)

B)

C)

D)

E) No reaction occurs.

A)

B)

C)

D)

E) No reaction occurs.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
23
Predict the major organic product and draw the structure of the electrophile in the following
reaction.
reaction.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
24
Explain why benzene is inert to treatment with hydrogen gas unless special conditions are used.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following statements about the reaction shown here is true?

A) The ortho and para pathways are disfavored because of steric hindrance.
B) Sulfuric acid and nitric acid in combination direct groups to the meta position on the ring.
C) The intermediate for the ortho and para pathways has positive and partial positive charges on adjacent atoms whereas the meta pathway intermediate does not.
D) Reaction produces benzoic action.
E) The reaction is a Friedel-Crafts acylation.

A) The ortho and para pathways are disfavored because of steric hindrance.
B) Sulfuric acid and nitric acid in combination direct groups to the meta position on the ring.
C) The intermediate for the ortho and para pathways has positive and partial positive charges on adjacent atoms whereas the meta pathway intermediate does not.
D) Reaction produces benzoic action.
E) The reaction is a Friedel-Crafts acylation.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
26
Draw the structure of the electrophile in the reaction shown. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
27
Draw an energy diagram for the chlorination of benzene.Include all intermediates and show their
relative energies.
relative energies.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
28
Bromination of aniline results in 2,4,6 -tribromoaniline. Which of the following compounds will be mono-brominated in para position upon treatment with  and a Lewis acid catalyst?
and a Lewis acid catalyst?
A)

B)

C)

D)

E)

 and a Lewis acid catalyst?
and a Lewis acid catalyst?A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following compounds will undergo nucleophilic aromatic substitution with methoxide ion at the fastest rate?
A)

B)1
1ec7e89_7ac1_e6f8_9927_f7309a5170d9_TB34225555_11
C)

D)

E)

A)

B)1
1ec7e89_7ac1_e6f8_9927_f7309a5170d9_TB34225555_11
C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following compounds is the major product of this reaction?

A)

B)

C)

D)

E)


A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
31
Shown below is an electrophilic aromatic substitution reaction. Which of the following statements about this reaction is/are true?

I. The products of ortho and para substitution are favored in the overall reaction.
II. The product of meta substitution is favored in the overall reaction.
III. For all three substitution pathways (ortho, meta, and para) the transition state in the rate-limiting step is lower in energy than the transition state for rate-limiting step in the bromination of benzene.
IV. Compared to the energy of the transition state for the rate-limiting step in the bromination of benzene, the transition state in the rate-limiting step for this reaction is lower in energy for the ortho and para pathways, but higher in energy for the meta pathway.
A) I
B) II
C) I and III
D) I and IV
E) II and IV

I. The products of ortho and para substitution are favored in the overall reaction.
II. The product of meta substitution is favored in the overall reaction.
III. For all three substitution pathways (ortho, meta, and para) the transition state in the rate-limiting step is lower in energy than the transition state for rate-limiting step in the bromination of benzene.
IV. Compared to the energy of the transition state for the rate-limiting step in the bromination of benzene, the transition state in the rate-limiting step for this reaction is lower in energy for the ortho and para pathways, but higher in energy for the meta pathway.
A) I
B) II
C) I and III
D) I and IV
E) II and IV

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
32
Draw a mechanism for the transformation shown here.Include all lone pairs, curved arrows, and
nonzero formal charges.
nonzero formal charges.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
33
Predict the product of the following reaction. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following compounds is the major product of this reaction?

A)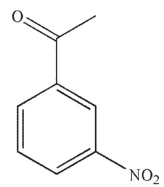
B)
C)
D)
E)

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
35
Draw a mechanism to illustrate the following transformation.Include all necessary lone pairs, curved arrows, and nonzero formal charges. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
36
Predict the product of the following reaction. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following compounds do you expect to most readily undergo Diels-Alder reaction with a dienophile? 
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
38
Draw the energy diagram for the following reaction. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
39
Draw a mechanism for the following transformation.Include all necessary lone pairs, curved arrows, and nonzero formal charges. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
40
What is the major product of the reaction shown here?

A)
B)
C)
D) Both a and c will form.
E) None of these products can form under these conditions.

A)

B)

C)

D) Both a and c will form.
E) None of these products can form under these conditions.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
41
What conditions are required to synthesize the molecule shown from benzene? 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
42
What is the product of the following reaction? 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
43
Aniline is more activating towards electrophilic aromatic substitution than phenol.Explain.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
44
Design a multistep synthesis for the following transformation.You may use any organic or inorganic reagents.Show the reagents needed for each step and the product of each step. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
45
A chemist attempted the following Friedel-Crafts alkylation and did not successfully isolate the intended product:  Explain what product the chemist most likely did isolate and why, and provide an alternate pathway starting from benzene that would provide the intended product.
Explain what product the chemist most likely did isolate and why, and provide an alternate pathway starting from benzene that would provide the intended product.
 Explain what product the chemist most likely did isolate and why, and provide an alternate pathway starting from benzene that would provide the intended product.
Explain what product the chemist most likely did isolate and why, and provide an alternate pathway starting from benzene that would provide the intended product.
Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
46
Phenol usually reacts easily with electrophiles.However, nitration of phenol with nitric acid is unsuccessful since the oxidation of phenol to p-quinone is faster than the electrophilic substitution.  However, the nitration reaction shown below is successful. Explain why the reactivity of starting material is different from that of phenol. Draw the nitration product.
However, the nitration reaction shown below is successful. Explain why the reactivity of starting material is different from that of phenol. Draw the nitration product. 
 However, the nitration reaction shown below is successful. Explain why the reactivity of starting material is different from that of phenol. Draw the nitration product.
However, the nitration reaction shown below is successful. Explain why the reactivity of starting material is different from that of phenol. Draw the nitration product. 

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
47
Predict the product of the following reaction and show the product after the first step. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
48
Draw a mechanism for the following transformation.Include all necessary lone pairs, curved arrows, and nonzero formal charges. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
49
Design a multistep synthesis for the transformation shown using a Friedel-Crafts acylation as one of the steps.You may use any organic or inorganic reagents.Show the reagents needed for each step and the product of each step. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
50
Predict the major organic product and draw the structure of the electrophile in the reaction shown here. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
51
Draw a mechanism to illustrate the following transformation.Include all necessary lone pairs, curved arrows, and nonzero formal charges. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
52
Draw the structure of the electrophile in the following reaction. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
53
Predict the major product of the following reaction. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
54
Predict the product of the following reaction and draw a mechanism to rationalize its formation. Include all necessary lone pairs, curved arrows, and nonzero formal charges. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
55
Benzene is subjected to an electrophilic aromatic substitution reaction, yielding a single product. This product is then subjected to the same reaction again.The reaction is successful, though slower than the first, and two products result.One of these, product A, has two signals in the aromatic region of the 1H NMR spectrum, while the other, product B, has only one signal.Propose possible structures for A and B.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
56
Predict the product of the following reaction. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
57
Provide the missing reagents in this scheme. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
58
Design a multistep synthesis for the following transformation.You may use any organic or inorganic reagents.Show the reagents needed for each step and the product of each step. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
59
Predict the product of the reaction shown below and draw a mechanism to rationalize its formation.Include all necessary lone pairs, curved arrows, and nonzero formal charges. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
60
Draw a mechanism for the following transformation.Include all necessary lone pairs, curved arrows, and nonzero formal charges. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
61
Explain why furan undergoes the Diels-Alder reaction, but benzene typically does not.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
62
The following reaction yields two products.Draw their structures. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
63
Design a multistep synthesis to produce o-bromoaniline as the sole product using aniline as the
starting material (i.e., you must avoid generating over-brominated products and p-bromoaniline).
You may use any organic or inorganic reagents.Show the reagents needed for each step and the
product of each step.
starting material (i.e., you must avoid generating over-brominated products and p-bromoaniline).
You may use any organic or inorganic reagents.Show the reagents needed for each step and the
product of each step.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
64
Predict the product of the following reaction. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
65
What is the product of the following reaction? 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
66
Design a multistep synthesis for the following transformation.You may use any organic or
inorganic reagents.Show the reagents needed for each step and the product of each step.
inorganic reagents.Show the reagents needed for each step and the product of each step.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
67
What is the product of the following reaction? 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
68
Design a synthesis of the target molecule shown from benzene and any organic or inorganic
reagents.Show the reagents needed for each step and the product of each step.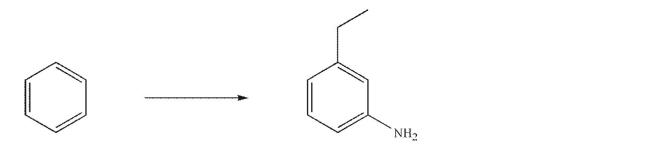
reagents.Show the reagents needed for each step and the product of each step.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck


