Deck 14: Functional Derivatives of Carboxylic Acids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/59
Play
Full screen (f)
Deck 14: Functional Derivatives of Carboxylic Acids
1
Which is the order of increasing boiling point of the following compounds (lowest first)? 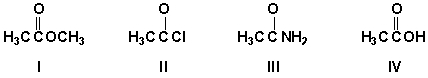
A) IV, I, III, II
B) I, III, IV, II
C) II, I, IV, III
D) IV, III, II, I
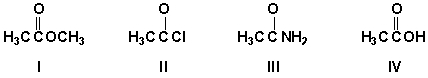
A) IV, I, III, II
B) I, III, IV, II
C) II, I, IV, III
D) IV, III, II, I
II, I, IV, III
2
Which is the final product of a series of reactions starting with ethyl benzoate and reacting with 1) aqueous sulfuric acid and heat, 2) thionyl chloride, and 3) ethylamine? 
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV
II
3
Why are the amino acids in proteins connected by means of amide and not ester bonds?
(I) Amides are more stable than esters.
(II) Esters are more stable than amides.
(III) Amides can be easily transformed into carboxylic acids and amines.
(IV) Amides are resistant to hydrolysis in the absence of enzymes.
A) I, III
B) I, II
C) I, IV
D) II, III
(I) Amides are more stable than esters.
(II) Esters are more stable than amides.
(III) Amides can be easily transformed into carboxylic acids and amines.
(IV) Amides are resistant to hydrolysis in the absence of enzymes.
A) I, III
B) I, II
C) I, IV
D) II, III
I, IV
4
Characterize the amides I and II in caffeine. 
A) I is a primary amide, II is a tertiary amide
B) I is a secondary amide, II is a secondary amide
C) I is a tertiary amide, II is a secondary amide
D) I is a tertiary amide, II is a tertiary amide

A) I is a primary amide, II is a tertiary amide
B) I is a secondary amide, II is a secondary amide
C) I is a tertiary amide, II is a secondary amide
D) I is a tertiary amide, II is a tertiary amide

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
5
Carboxylic acids and amides have in general higher boiling points than esters and anhydrides because of which property?
A) dispersion forces
B) resonance stabilization
C) conjugated functional groups
D) hydrogen bonding
A) dispersion forces
B) resonance stabilization
C) conjugated functional groups
D) hydrogen bonding

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
6
Partial hydrolysis of phenobarbitol gives which compounds? 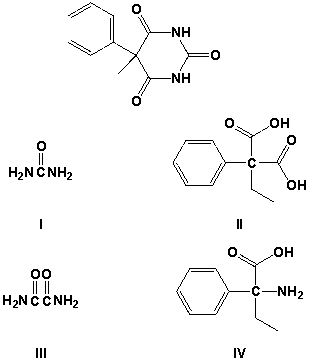
A) I, II
B) II, III
C) III, IV
D) I, III

A) I, II
B) II, III
C) III, IV
D) I, III

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
7
Which compounds will yield benzoic acid when hydrolyzed?
(I) benzyl ethanoate
(II) benzamide
(III) phenyl ethanoate
(IV) methyl benzoate
A) I, II
B) III, IV
C) I, III
D) II, IV
(I) benzyl ethanoate
(II) benzamide
(III) phenyl ethanoate
(IV) methyl benzoate
A) I, II
B) III, IV
C) I, III
D) II, IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
8
Which is the product of the reaction of phthalic anhydride with 1 equivalent of methanol? 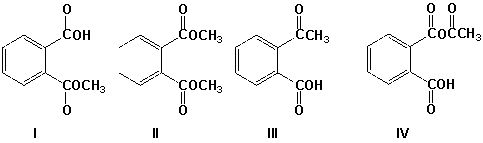
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
9
Which reactions can be used to prepare an ester? 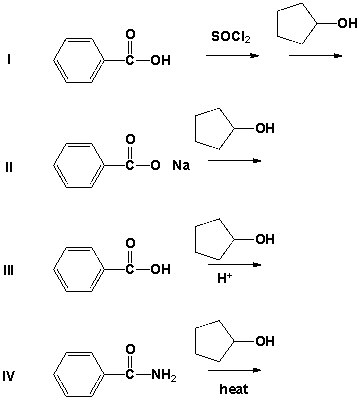
A) II, IV
B) I, III
C) I, II
D) III, IV

A) II, IV
B) I, III
C) I, II
D) III, IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
10
What is the order of decreasing reactivity toward nucleophilic acyl substitution for these carboxylic acid derivatives (most reactive first)? 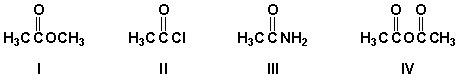
A) II, IV, I, III
B) III, IV, I, II
C) IV, I, II, III
D) I, II, III, IV
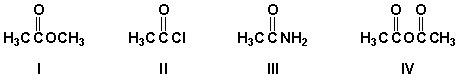
A) II, IV, I, III
B) III, IV, I, II
C) IV, I, II, III
D) I, II, III, IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
11
Which is the correct structure for phenyl benzoate? 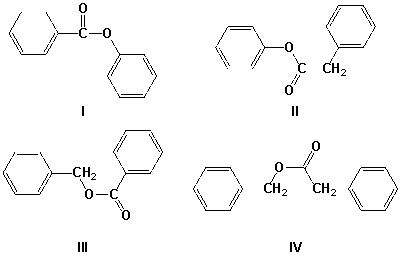
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
12
Which are the best conditions for the following preparation? 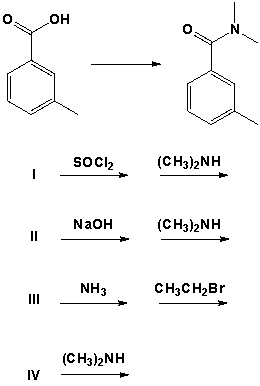
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
13
Which compounds are named correctly? 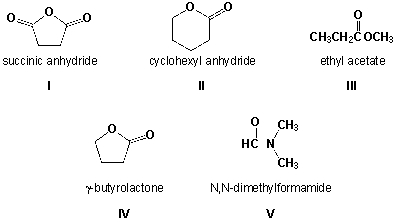
A) I, II, III
B) II, III, IV
C) I, IV, V
D) III, IV, V

A) I, II, III
B) II, III, IV
C) I, IV, V
D) III, IV, V

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
14
Which is the common name for the following compound? 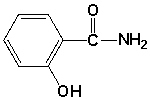
A) aspirin
B) cyclosporin
C) succinamide
D) salicylamide

A) aspirin
B) cyclosporin
C) succinamide
D) salicylamide

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
15
Which functional groups are correctly named? 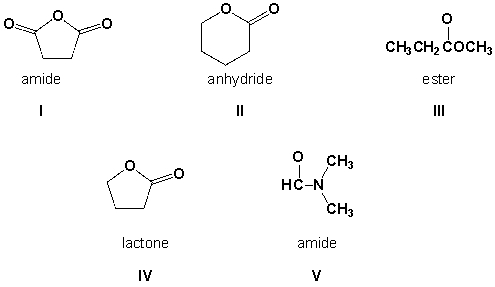
A) I, II, III, IV
B) I, III, IV, V
C) III, IV, V
D) I, II, IV, V

A) I, II, III, IV
B) I, III, IV, V
C) III, IV, V
D) I, II, IV, V

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
16
Which is the major product when 1,2-diaminoethane is heated with dimethyl oxalate? 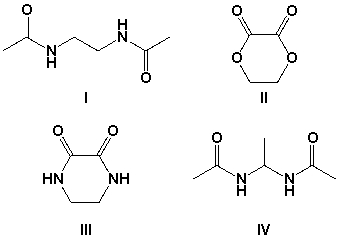
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
17
Penicillins derived from Penicillium fungi were the first available -lactam antibiotics, which inhibit the biosynthesis of the peptidoglycan layer of bacterial cell walls. Which detail of the structure of penicillin is the "lactam-unit"? 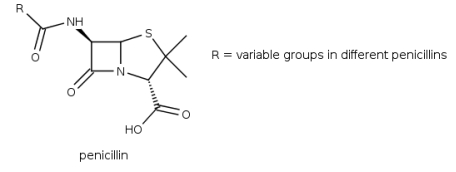
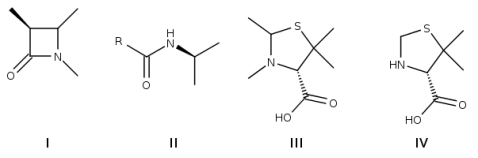
A) I
B) II
C) III
D) IV


A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
18
Which is the major product of the reaction of 4-aminophenol with 1 equivalent of acetic anhydride? 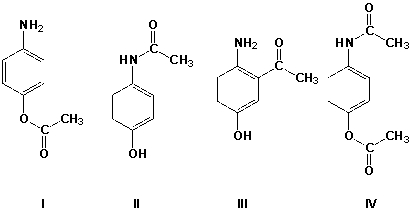
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following compounds does not yield nicotinamide when reacted with ammonia at room temperature? 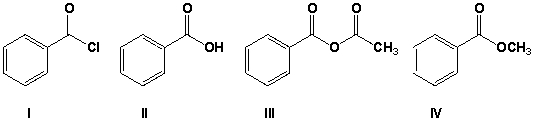
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
20
The following reaction is fastest when Z is which group? 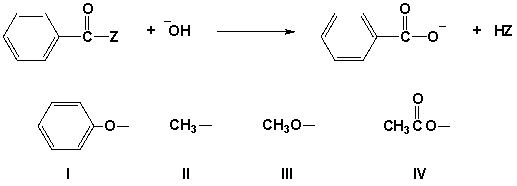
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
21
The reagent that complete the following reaction is, 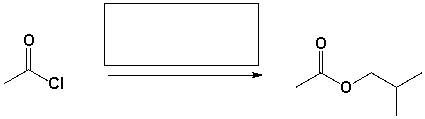


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
22
Chitin is the main component of the cell walls of fungi, the exoskeletons of arthropods and insects and the radula of mollusks. Which statements are true? 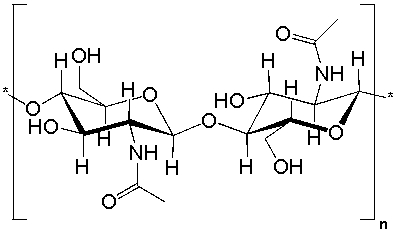 I) Chitin is made from an aminosugar.
I) Chitin is made from an aminosugar.
II) Chitin contains amide bonds.
III) Chitin is a macromolecule
IV) Chitin is structurally related to cellulose
A) all of the above
B) I, II, and IV
C) I, II, and III
D) II, III, and IV
 I) Chitin is made from an aminosugar.
I) Chitin is made from an aminosugar.II) Chitin contains amide bonds.
III) Chitin is a macromolecule
IV) Chitin is structurally related to cellulose
A) all of the above
B) I, II, and IV
C) I, II, and III
D) II, III, and IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
23
The reagents that complete the following reaction are, 


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
24
The reagents that complete the following reaction are, 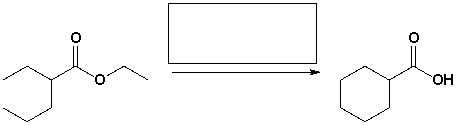


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
25
Butyrolactone reacts with 2-methylpropan-1-amine in DMF (dimethylformamide). Which is the major product? 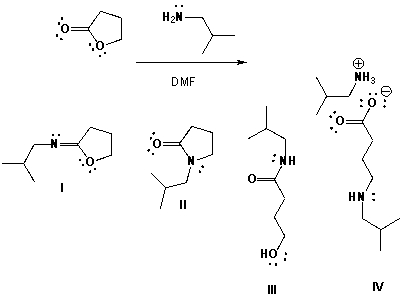
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
26
Which reactions proceed at room temperature as written? 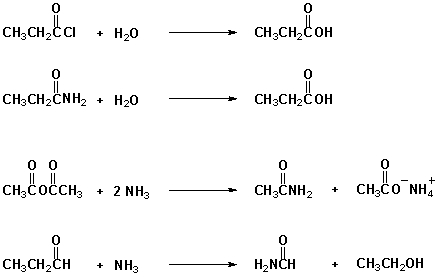
A) I, II
B) I, III
C) II, III
D) II, IV

A) I, II
B) I, III
C) II, III
D) II, IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
27
Butyrolactone reacts with isobutyl magnesium bromide. Which is the major product formed? 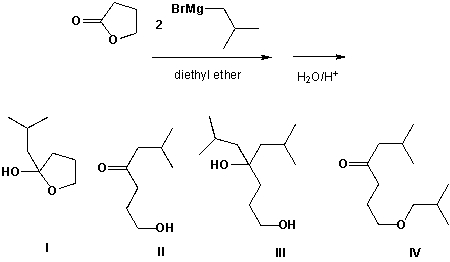
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
28
The major product that completes the following reaction is, 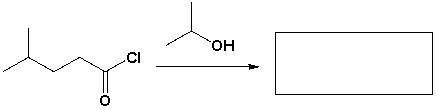


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
29
Which is the product from the following reaction? 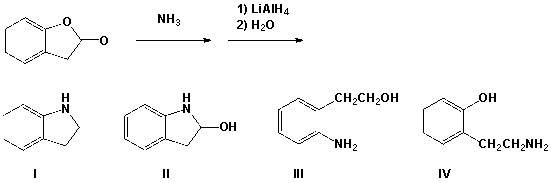
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
30
Which are the best conditions for the following reaction? 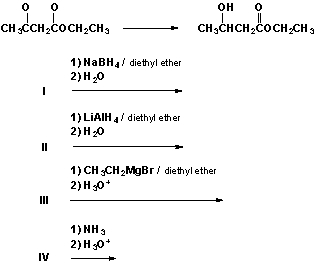
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
31
Which is the product from the reaction of sodium benzoate and acetyl chloride? 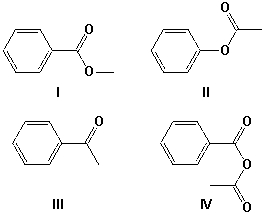
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
32
The major product that completes the following reaction is, 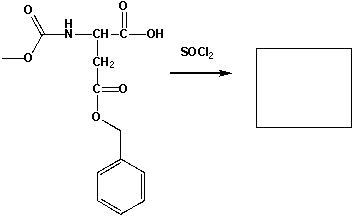


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
33
What are the major products of the following reaction?

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
34
Which are the best conditions for the following reaction? 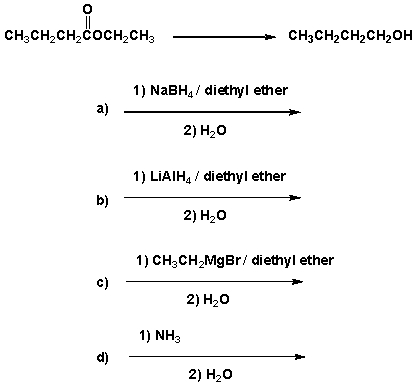
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
35
Which is the major product of the following reaction? 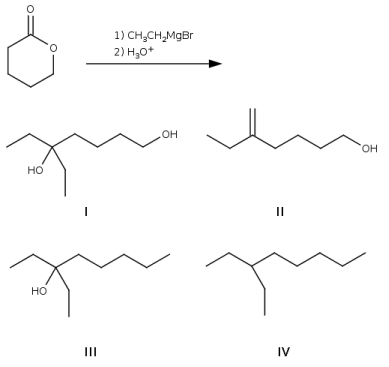
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
36
Which is the product of the reaction of ethyl benzoate with 2 equivalents of methyl Grignard followed by aqueous acid? 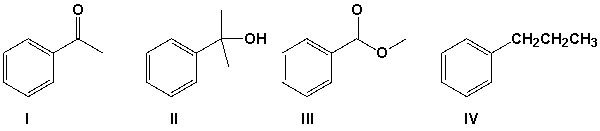
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
37
The starting material that completes the following reaction is, 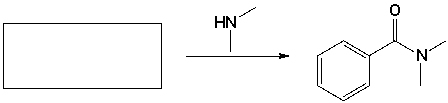


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
38
The IUPAC name of the following compound is ______________________________. 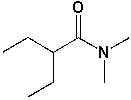


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
39
The IUPAC name of the following compound is ______________________________. 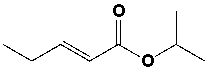


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
40
The major product that completes the following reaction is, 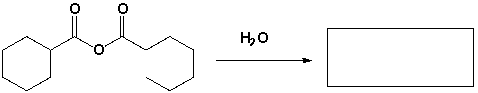


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
41
The product of the reaction of 5-pentanolactam with aqueous acid and heat is 5-aminopentanoic acid.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
42
Sodium borohydride reduces propanamide to propanamine.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
43
The order of reactivity of the carboxylic acid derivatives with water is, 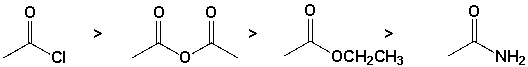


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
44
Complete the following reaction 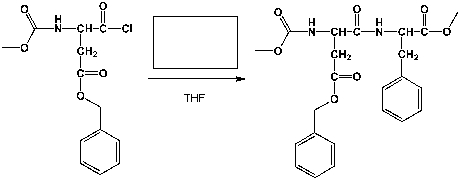


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
45
1 Mol of 5-hydroxyheptanoic acid reacts with 1 mol of SOCl2 and then with 1 mol of dimethylamine. What major products are formed in each step? 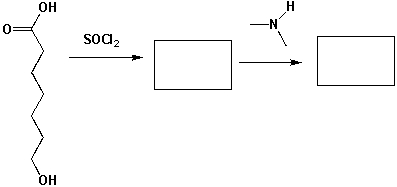


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
46
What is the reaction mechanism of the basic hydrolysis of ethyl-benzoate? 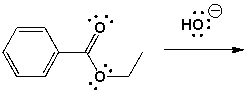


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
47
Complete the following reaction sequence. 


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
48
The product of the reaction of 4-butanolactone with ethanol and acid is butanoic acid and diethylether.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
49
The structure for cyclohexanecarboxylic 2,2-dimethylpropanoic anhydride is, 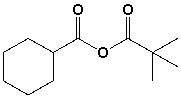


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
50
Which is the major product formed? 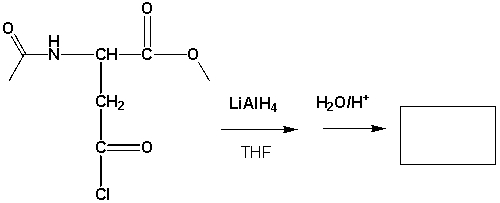


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
51
The proper mechanism for the reaction of acetyl chloride and water is shown below. 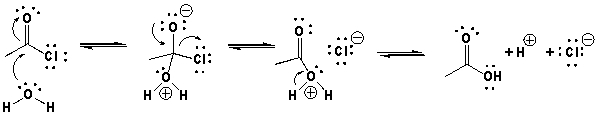


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
52
Triethoxymethane reacts with an aqueous solution of sodium carbonate. What are the reaction products? 


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
53
The reagents that complete the following reaction are, 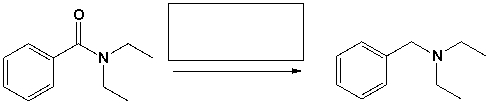


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
54
The product of the reaction of propanamide with ethanol is ethyl propanoate.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
55
The order of reactivity of the carboxylic acid derivatives with ammonia is, 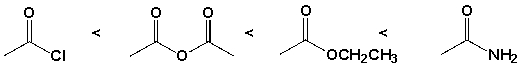


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
56
The name of the following compound is 3-methylbutanoyl 2-methylbutanoate. 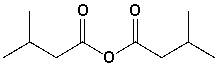


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
57
The major product that completes the following reaction is 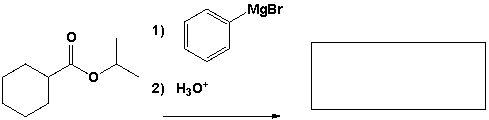


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
58
1 Mol of 5-hydroxypentanoic acid reacts with 1 mol of SOCl2 and then with 1 mol of dimethylamine. What major products are formed in each step? 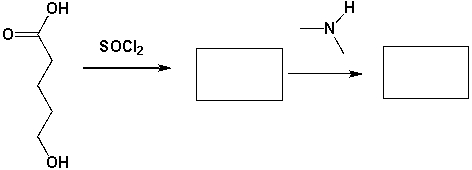


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
59
Lithium aluminum hydride reduces 3-oxobutanoic acid to 4-hydroxy-2-butanone.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck


