Deck 11: Infrared and Nuclear Magnetic Resonance Spectroscopy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/117
Play
Full screen (f)
Deck 11: Infrared and Nuclear Magnetic Resonance Spectroscopy
1
How many principal vibrations does benzene (C6H6) have?
A) 30
B) 29
C) 18
D) 17
A) 30
B) 29
C) 18
D) 17
30
2
Very prominent peaks in the IR spectrum are sufficient to distinguish between which compounds?
I. butanoic acid and methyl butanoate
II. dimethylamine and N,N-dimethylformamide
III. triethylamine and dimethylpropanamine
IV. 2-pentanol and 3-pentanol
A) I, II
B) II, III
C) I, IV
D) III, IV
I. butanoic acid and methyl butanoate
II. dimethylamine and N,N-dimethylformamide
III. triethylamine and dimethylpropanamine
IV. 2-pentanol and 3-pentanol
A) I, II
B) II, III
C) I, IV
D) III, IV
I, II
3
Which is the approximate energy range for absorptions in the infrared region of the electromagnetic spectrum?
A) 0.042 to 0.42 kJ/mol
B) 0.042 to 0.42 kW/mol
C) 420 to 2,100 kJ/mol
D) 8.4 to 41.9 kJ/mol
A) 0.042 to 0.42 kJ/mol
B) 0.042 to 0.42 kW/mol
C) 420 to 2,100 kJ/mol
D) 8.4 to 41.9 kJ/mol
8.4 to 41.9 kJ/mol
4
How many principal vibrations does aspartame have? 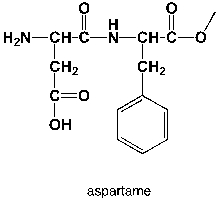
A) 99
B) 141
C) 112
D) 111

A) 99
B) 141
C) 112
D) 111

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
5
Which is the order of increasing bond stretching frequency (lowest first)?
I. C=N
II. C=O
III. C=C
IV. C=S
A) II, I, IV, III
B) I, II, IV, III
C) III, I, II, IV
D) IV, III, I, II
I. C=N
II. C=O
III. C=C
IV. C=S
A) II, I, IV, III
B) I, II, IV, III
C) III, I, II, IV
D) IV, III, I, II

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
6
Which region in the IR spectrum could be used to distinguish between naproxen and diphenhydramine (Benadryl)? 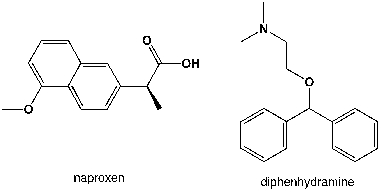
A) 3000-3050 cm-1
B) 1360-1380 cm-1
C) 3100-3500 cm-1
D) 2400-3500 cm-1

A) 3000-3050 cm-1
B) 1360-1380 cm-1
C) 3100-3500 cm-1
D) 2400-3500 cm-1

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
7
A compound has the molecular formula C5H10O, and strong absorptions at 1100 and 3350 cm-1. Which is the most likely structure for the compound? 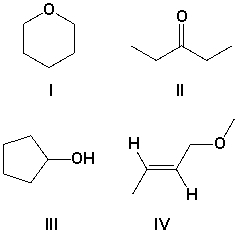
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
8
Which is the wavelength ( m) of an infrared absorption band at 500 cm-1?
A) 0.2
B) 2.0
C) 20
D) 25
A) 0.2
B) 2.0
C) 20
D) 25

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
9
The broadening of the stretching vibration peak for alcohols in the liquid or solid phase is mainly due to which of the following?
A) water contamination
B) a strong dipole moment
C) hydrogen bonding
D) coupling peaks
A) water contamination
B) a strong dipole moment
C) hydrogen bonding
D) coupling peaks

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
10
Which is the index of hydrogen deficiency for a compound having molecular formula C7H5Cl2NO?
A) 4
B) 5
C) 6
D) 7
A) 4
B) 5
C) 6
D) 7

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
11
Which is the approximate energy range for absorptions in the infrared region of the electromagnetic spectrum?
A) 50 to 100 kcal/mol
B) 10 to 50 kcal/mol
C) 2 to 10 kcal/mol
D) 0.01 to 0.1 kcal/mol
A) 50 to 100 kcal/mol
B) 10 to 50 kcal/mol
C) 2 to 10 kcal/mol
D) 0.01 to 0.1 kcal/mol

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
12
Prominent peaks in the IR spectrum are sufficient to distinguish between which compounds?
I. diethyl ether and diethylamine
II. cyclohexanone and cyclopentanone
III. pentanal and pentanol
IV. 2-hexene and 3-hexene
A) I, III
B) II, III
C) II, IV
D) III, IV
I. diethyl ether and diethylamine
II. cyclohexanone and cyclopentanone
III. pentanal and pentanol
IV. 2-hexene and 3-hexene
A) I, III
B) II, III
C) II, IV
D) III, IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
13
Which is the wave number for an infrared band at 5 microns ( m)?
A) 500 cm-1
B) 2000 cm-1
C) 5000 cm-1
D) 10,000 cm-1
A) 500 cm-1
B) 2000 cm-1
C) 5000 cm-1
D) 10,000 cm-1

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
14
The stretching vibration peak for alcohols is very sharp when the spectrum is recorded in the gas phase. This is mainly due to the following:
A) lower concentration
B) absence of hydrogen bonding
C) higher degree of hydrogen bonding
D) higher temperature
A) lower concentration
B) absence of hydrogen bonding
C) higher degree of hydrogen bonding
D) higher temperature

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
15
Which compounds will have a particularly weak characteristic IR stretching frequency? 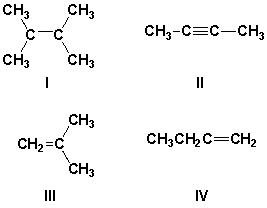
A) I, II
B) III, IV
C) I, III
D) II, IV

A) I, II
B) III, IV
C) I, III
D) II, IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
16
How many principal vibrations does anthracene have? 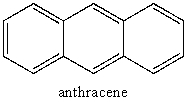
A) 36
B) 37
C) 66
D) 67

A) 36
B) 37
C) 66
D) 67

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
17
Which is the index of hydrogen deficiency for a compound having molecular formula C12H13Br2NO2?
A) 5
B) 6
C) 7
D) 8
A) 5
B) 6
C) 7
D) 8

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
18
Which region in the IR spectrum could be used to distinguish between the neurotransmitters acetylcholine and 4-aminobutanoic acid ? 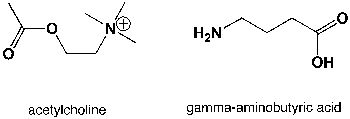
A) 2900-3000 cm-1
B) 1360-1380 cm-1
C) 3100-3500 cm-1
D) 2400-3500 cm-1

A) 2900-3000 cm-1
B) 1360-1380 cm-1
C) 3100-3500 cm-1
D) 2400-3500 cm-1

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
19
Which is the order of increasing bond stretching frequency (lowest first)?
I. C-H
II. N-H
III. O-H
IV. F-H
A) I, II, III, IV
B) I, III, II, IV
C) IV, III, II, I
D) III, II, I, IV
I. C-H
II. N-H
III. O-H
IV. F-H
A) I, II, III, IV
B) I, III, II, IV
C) IV, III, II, I
D) III, II, I, IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
20
What is the general formula for the calculation of the number of principal vibrations of a molecule that extends in all three directions (e.g. cyclohexane)? N is the number of atoms that form the molecule.
A) 3N
B) 3N-6
C) 3N-3
D) 3N-5
A) 3N
B) 3N-6
C) 3N-3
D) 3N-5

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
21
Prominent peaks in the IR spectrum are sufficient to distinguish between which compounds?
I. butane and cyclobutane
II. toluene and methylcyclohexane
III. 2-pentanone and 3-pentanone
IV. pentanal and 1-pentene
A) I, III
B) II, III
C) II, IV
D) III, IV
I. butane and cyclobutane
II. toluene and methylcyclohexane
III. 2-pentanone and 3-pentanone
IV. pentanal and 1-pentene
A) I, III
B) II, III
C) II, IV
D) III, IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
22
How many states in a static magnetic field can be discerned for an NMR-active isotope with a spin of 1 (e.g. 2D)?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
23
Which compound has characteristic IR peaks at 3100 cm-1 and 1650 cm-1, but not 1480 cm-1? 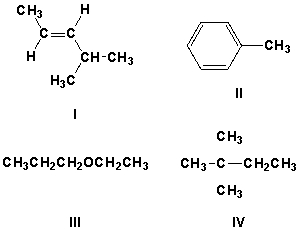
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
24
Which compound has a sharp IR absorption at 1710 cm-1 and a very broad band at 3300 cm-1? 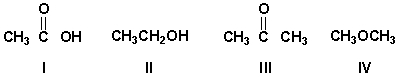
A) I
B) II
C) III
D) IV
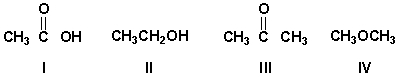
A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
25
How many sets of equivalent hydrogen atoms are there for 2-propanol?
A) 2
B) 3
C) 4
D) 8
A) 2
B) 3
C) 4
D) 8

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
26
Which compound has characteristic IR peaks at 3100 cm-1, 1600 cm-1, and 1475 cm-1? 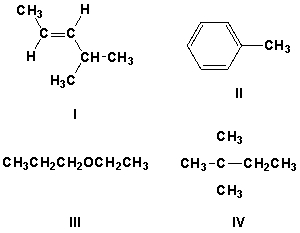
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
27
Which region in the IR spectrum could be used to distinguish between aspirin and acetaminophen (tylenol)? 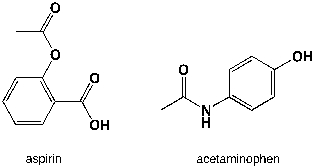
A) 3000-3050 cm-1
B) 1360-1380 cm-1
C) 3100-3500 cm-1
D) 2400-3500 cm-1

A) 3000-3050 cm-1
B) 1360-1380 cm-1
C) 3100-3500 cm-1
D) 2400-3500 cm-1

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
28
Which regions in the IR spectrum could be used to distinguish between benzene and cyclohexane?
A) 1360-1380 cm-1
B) 2900-3100 cm-1
C) 1680-1750 cm-1
D) 3200-3600 cm-1
A) 1360-1380 cm-1
B) 2900-3100 cm-1
C) 1680-1750 cm-1
D) 3200-3600 cm-1

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
29
A compound has a molecular formula of C5H9N and the IR spectrum has peaks in the region of 2900 to 3000 cm-1, 1450 cm-1 and 1375 cm-1, but no peaks in the region of 3000-3500 cm-1. Which of the following could be the compound? 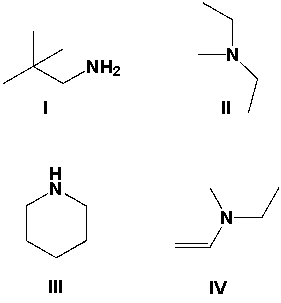
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
30
Which compound has a characteristic IR peak at 2900 cm-1? 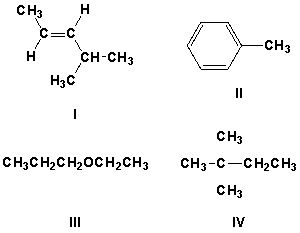
A) I, II, III
B) I, II, III, IV
C) III, IV
D) I, III, IV

A) I, II, III
B) I, II, III, IV
C) III, IV
D) I, III, IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
31
How many states in a static magnetic field can be discerned for an NMR-active isotope with a spin of 1/2 (e.g. 1H, 13C)?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
32
The chemical shift in NMR spectroscopy is observed, because________________________.
A) the magnetic field is not constant during the measurement
B) of the interfering magnetic field caused by the electrons in the molecule
C) many elements have more than one isotope
D) the interference of neighboring nuclei
A) the magnetic field is not constant during the measurement
B) of the interfering magnetic field caused by the electrons in the molecule
C) many elements have more than one isotope
D) the interference of neighboring nuclei

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
33
Which region in the IR spectrum could be used to distinguish between benzyl amine and benzamide?
A) 1475 cm-1 and 1600 cm-1
B) 3030 cm-1
C) 3100-3500 cm-1
D) 1630-1690 cm-1
A) 1475 cm-1 and 1600 cm-1
B) 3030 cm-1
C) 3100-3500 cm-1
D) 1630-1690 cm-1

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
34
Which region in the IR spectrum could be used to distinguish between aspirin and naproxen (aleve)? 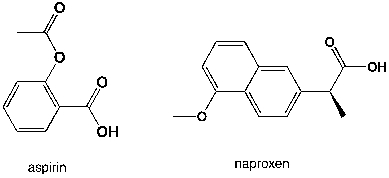
A) 2900-3000 cm-1
B) 1360-1380 cm-1
C) 1700-1800 cm-1
D) 3100-3500 cm-1

A) 2900-3000 cm-1
B) 1360-1380 cm-1
C) 1700-1800 cm-1
D) 3100-3500 cm-1

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
35
Which regions in the IR spectrum could be used to distinguish between butanoic acid and 2-butanone?
A) 3200-3600 cm-1
B) 1600 cm-1
C) 1750-1800 cm-1
D) 2500-3300 cm-1
A) 3200-3600 cm-1
B) 1600 cm-1
C) 1750-1800 cm-1
D) 2500-3300 cm-1

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
36
Which compound has an IR absorption at 3300 cm-1 but no band at 1710 cm-1? 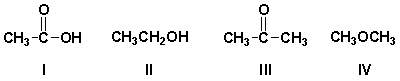
A) I
B) II
C) III
D) IV
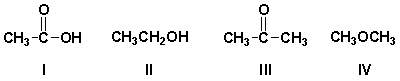
A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
37
How many states in a static magnetic field can be discerned for an NMR-active isotope with a spin of 3 (e.g. 10B)?
A) 6
B) 7
C) 8
D) 9
A) 6
B) 7
C) 8
D) 9

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
38
Which region in the IR spectrum could be used to distinguish between aspartame and saccharine? 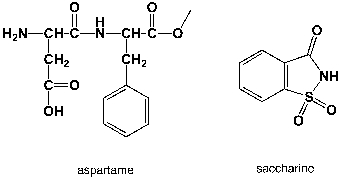
A) 3000-3050 cm-1
B) 1060-1100 cm-1
C) 1440-1465 cm-1
D) 2900-3000 cm-1

A) 3000-3050 cm-1
B) 1060-1100 cm-1
C) 1440-1465 cm-1
D) 2900-3000 cm-1

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
39
A compound has a molecular formula C7H14O2 and a broad signal in the region of 2850-3000 cm-1 and a signal in the region of 1710-1780 cm-1 in the IR spectrum. Which could be the compound?(Hint: There’s no broad absorption band near 3300 cm-1 in this spectrum..)
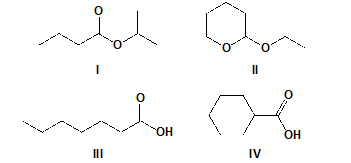
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
40
Which is the most likely structure for a compound with molecular formula C5H8O, and an IR spectrum with an intense peak at 1710 cm-1 but no peaks between 3000 and 3050 cm-1? 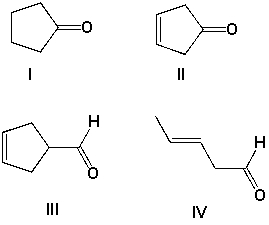
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
41
Which splitting pattern (Ha:Hb:Hc) is observed ? 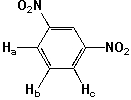
A) singlet:doublet:singlet
B) singlet:triplet:doublet
C) singlet:triplet:singlet
D) doublet:sixtet:doublet

A) singlet:doublet:singlet
B) singlet:triplet:doublet
C) singlet:triplet:singlet
D) doublet:sixtet:doublet

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
42
Which compound has a singlet in the 1H-NMR at approximately 2.0 ppm that integrates to 3 hydrogens? 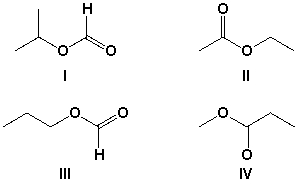
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
43
How many sets of equivalent hydrogen (H) and carbon (C) atoms are there for the following compound? 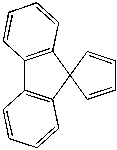
A) 6H, 9C
B) 12H, 17C
C) 6H, 17C
D) 8H, 9C

A) 6H, 9C
B) 12H, 17C
C) 6H, 17C
D) 8H, 9C

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
44
Which are the relative areas of integration in the 1H-NMR spectrum for 2-chloro-3-methylbutane?
A) 9:1:1
B) 3:3:3:1:1
C) 6:3:2
D) 6:3:1:1
A) 9:1:1
B) 3:3:3:1:1
C) 6:3:2
D) 6:3:1:1

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
45
How many sets of equivalent carbon atoms are there for the following compound (C16H26O)? 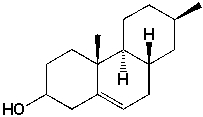
A) 12
B) 14
C) 16
D) 13

A) 12
B) 14
C) 16
D) 13

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
46
Which is the splitting pattern for the hydrogen atoms in 2,2-dibromopropane?
A) septet
B) quartet
C) doublet
D) singlet
A) septet
B) quartet
C) doublet
D) singlet

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
47
Which is the splitting pattern for the indicated hydrogen atom? 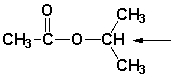
A) septet
B) quartet
C) doublet
D) singlet

A) septet
B) quartet
C) doublet
D) singlet

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
48
Which are the characteristic shift ranges for the indicated hydrogen atoms? 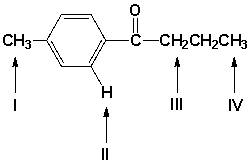
A) I = 0.8-1.0, II = 6.5-8.5, III = 2.2-2.5, IV = 2.1-2.3
B) I = 0.8-1.0, II = 2.2-2.5, III = 6.5-8.5, IV = 2.1-2.3
C) I = 2.2-2.5, II = 6.5-8.5, III = 0.8-1.0, IV = 2.1-2.3
D) I = 2.2-2.5, II = 6.5-8.5, III = 2.1-2.3, IV = 0.8-1.0

A) I = 0.8-1.0, II = 6.5-8.5, III = 2.2-2.5, IV = 2.1-2.3
B) I = 0.8-1.0, II = 2.2-2.5, III = 6.5-8.5, IV = 2.1-2.3
C) I = 2.2-2.5, II = 6.5-8.5, III = 0.8-1.0, IV = 2.1-2.3
D) I = 2.2-2.5, II = 6.5-8.5, III = 2.1-2.3, IV = 0.8-1.0

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
49
Which is the order of increasing values in the 13C NMR spectra for the following carbon atoms?
CH4 CH3F CH3Br CH3OH CH3Cl
I II III IV V
A) III, V, II, IV, I
B) I, IV, II, V, III
C) V, IV, II, III, I
D) I, III, V, IV, II
CH4 CH3F CH3Br CH3OH CH3Cl
I II III IV V
A) III, V, II, IV, I
B) I, IV, II, V, III
C) V, IV, II, III, I
D) I, III, V, IV, II

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
50
Which compound has four sets of signals in the 1H-NMR spectrum? 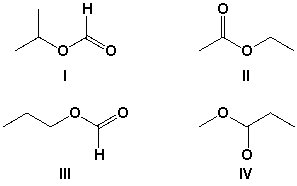
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
51
Which are the relative areas of integration in the 1H-NMR spectrum for the compound shown below? 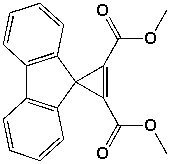
A) 3:1:1:1
B) 3:1:1:1:1
C) 3:1:1:1:1:1
D) 6:2:2:2

A) 3:1:1:1
B) 3:1:1:1:1
C) 3:1:1:1:1:1
D) 6:2:2:2

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
52
Which is the order in increasing chemical shift values in the 1H-NMR spectrum for the indicated hydrogen atoms (farthest upfield first)? 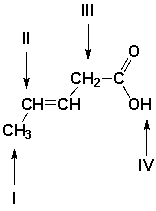
A) IV, II, III, I
B) I, III, II, IV
C) III, I, IV, II
D) II, IV, I, III

A) IV, II, III, I
B) I, III, II, IV
C) III, I, IV, II
D) II, IV, I, III

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
53
Which is the order of increasing values downfield from TMS for the methyl groups shown (farthest upfield first)?
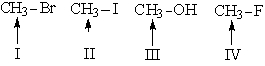
A) I, III, IV, II
B) II, I, III, IV
C) IV, II, III, I
D) III, IV, II, I
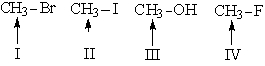
A) I, III, IV, II
B) II, I, III, IV
C) IV, II, III, I
D) III, IV, II, I

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
54
A compound gave two sets of signals in the 1H-NMR spectrum with an integration ratio of 60 to 10. Which is the most likely structure for the compound? 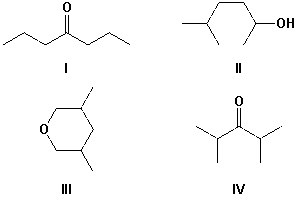
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
55
How many sets of equivalent hydrogen atoms are there for the following compound? 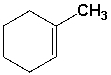
A) 3
B) 4
C) 5
D) 6

A) 3
B) 4
C) 5
D) 6

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
56
The splitting pattern for a compound with molecular formula C3H5OCl gave a quartet and a triplet. Which is the most likely structure for this compound? 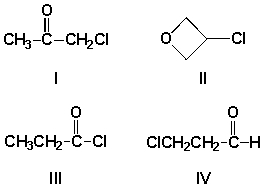
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
57
How many sets of signals are there in the 1H-NMR spectrum for diisopropyl ether?
A) 1
B) 2
C) 3
D) 6
A) 1
B) 2
C) 3
D) 6

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
58
Sugar Cane is a so-called C4 plant, a plant having a different mechanism of carbon fixation (binding of atmospheric carbon) than other plants. Consequently, the 13C-content of C4 plants is enriched, relative to non-C4 plants. This biochemical finding can be used to detect the amount of sugar from sugarcane, which has been added to the grape juice before its fermentation, by measuring the 13C-isotope content in the resulting ethanol. How can you measure the 13C-content of ethanol in a wine-sample?
A) "chemical shift"
B) "integration of the signal using a sample with known 13C-content of ethanol as standard"
C) "1H-1H signal splitting"
D) "13C-13C signal splitting"
A) "chemical shift"
B) "integration of the signal using a sample with known 13C-content of ethanol as standard"
C) "1H-1H signal splitting"
D) "13C-13C signal splitting"

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
59
Which compounds show signal splitting in the 1H-NMR spectrum? 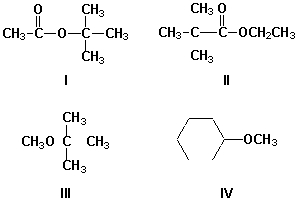
A) I, II
B) III, IV
C) I, III
D) II, IV

A) I, II
B) III, IV
C) I, III
D) II, IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
60
How many sets of signals are there in the 1H-NMR spectrum for 1,1-dichlorocyclohexane?
A) 2
B) 3
C) 4
D) 6
A) 2
B) 3
C) 4
D) 6

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
61
The energy in kJ/mol of 3350 cm-1 light is ___________________.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
62
How many sets of inequivalent carbon atoms are there for 1-bromo-3-chlorocyclohexane?
A) 3
B) 4
C) 5
D) 6
A) 3
B) 4
C) 5
D) 6

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
63
A compound has three signals in the 13C NMR spectrum and two signals in the 1H-NMR spectrum. Which is most likely the compound?
A) bromobenzene
B) para dibromobenzene
C) ortho dibromobenzene
D) meta dibromobenzene
A) bromobenzene
B) para dibromobenzene
C) ortho dibromobenzene
D) meta dibromobenzene

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
64
Testosterone is an anabolic steroid; estradiol is the predominant female sex hormone. How can you discern between these two hormones by using NMR spectroscopy? 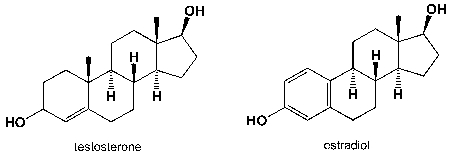 I) number of 13C-signals
I) number of 13C-signals
II) number of 1H-signals
III) chemical shifts
IV) 1H-signal splitting
A) I, II, III
B) I, II, III, IV
C) I, III, IV
D) II, III, IV
 I) number of 13C-signals
I) number of 13C-signalsII) number of 1H-signals
III) chemical shifts
IV) 1H-signal splitting
A) I, II, III
B) I, II, III, IV
C) I, III, IV
D) II, III, IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
65
The compounds shown below can be distinguished by which 1H-NMR characteristics? 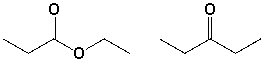 I) number of signals
I) number of signals
II) splitting
III) integration
IV) chemical shift
A) I, II, III
B) I, III, IV
C) I, II
D) IV
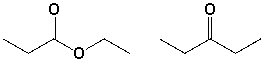 I) number of signals
I) number of signalsII) splitting
III) integration
IV) chemical shift
A) I, II, III
B) I, III, IV
C) I, II
D) IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
66
Which statement is wrong? 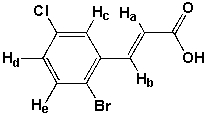
A) Ha and Hb are doublets
B) Hc is a doublet
C) Hd is a doublet
D) He is a doublet

A) Ha and Hb are doublets
B) Hc is a doublet
C) Hd is a doublet
D) He is a doublet

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
67
Which compounds have 3 signals in the 13C NMR spectrum? 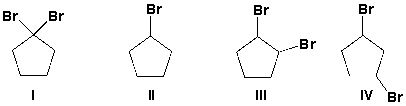
A) I, II
B) I, II, III
C) I, III
D) I, II, III, IV

A) I, II
B) I, II, III
C) I, III
D) I, II, III, IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
68
Which statement is wrong? 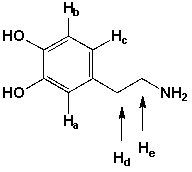
A) Ha and Hb are doublets
B) Hc is a doublet
C) Hd is a triplet
D) He is a triplet

A) Ha and Hb are doublets
B) Hc is a doublet
C) Hd is a triplet
D) He is a triplet

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
69
A compound has two signals in the 13C NMR spectrum and a single signal in the 1H-NMR spectrum. Which is most likely the compound?
A) dimethyl ether
B) diethyl ether
C) 2,2-dimethylpropane
D) methyl ethanoate
A) dimethyl ether
B) diethyl ether
C) 2,2-dimethylpropane
D) methyl ethanoate

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
70
The energy in kcal/mol of 606.06 m light is ___________________.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
71
How many sets of inequivalent carbon atoms are there for the following compound? 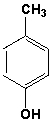
A) 3
B) 4
C) 5
D) 6

A) 3
B) 4
C) 5
D) 6

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
72
The molecules shown below are enantiomers. How do they differ in their 1H-NMR spectra? 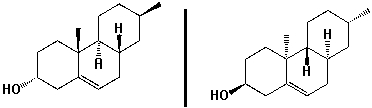
A) chemical shifts
B) signal splitting
C) integration
D) none of the above

A) chemical shifts
B) signal splitting
C) integration
D) none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
73
The energy in kcal/mol of 3350 cm-1 light is ___________________.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
74
The compounds shown below can be distinguished by which 1H-NMR characteristics? 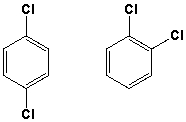 I) number of signals
I) number of signals
II) splitting
III) integration
IV) chemical shift
A) I, III, IV
B) I, II, IV
C) I, II, III
D) II, III, IV
 I) number of signals
I) number of signalsII) splitting
III) integration
IV) chemical shift
A) I, III, IV
B) I, II, IV
C) I, II, III
D) II, III, IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
75
How many sets of equivalent hydrogen (H) and carbon (C) atoms are there for the following compound? 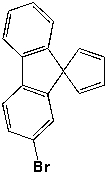
A) 5H, 9C
B) 11H, 17C
C) 5H, 17C
D) 7H, 9C

A) 5H, 9C
B) 11H, 17C
C) 5H, 17C
D) 7H, 9C

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
76
The ___________________functional group has a characteristic absorption in the region of 1630-1800 cm-1.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
77
A compound has a single signal in the 13C NMR spectrum and a single signal in the 1H-NMR spectrum. Which is most likely the compound?
A) dimethyl ether
B) diethyl ether
C) 2,2-dimethylpropane
D) methyl ethanoate
A) dimethyl ether
B) diethyl ether
C) 2,2-dimethylpropane
D) methyl ethanoate

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
78
The compounds shown below can be distinguished by which 1H-NMR characteristics? 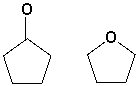 I) number of signals
I) number of signals
II) splitting
III) integration
IV) chemical shift
A) I, II, IV
B) I, IV
C) II
D) IV
 I) number of signals
I) number of signalsII) splitting
III) integration
IV) chemical shift
A) I, II, IV
B) I, IV
C) II
D) IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
79
The ___________________functional group has a characteristic absorption in the region of 3200-3500 cm-1.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
80
The molecules shown below are diastereomers. How do they differ in their 1H-NMR spectra? 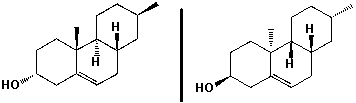
A) chemical shifts
B) signal splitting
C) integration
D) none of the above

A) chemical shifts
B) signal splitting
C) integration
D) none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck


