Deck 10: Amines
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/55
Play
Full screen (f)
Deck 10: Amines
1
Arrange the basicities of the four amines in decreasing order (highest first).
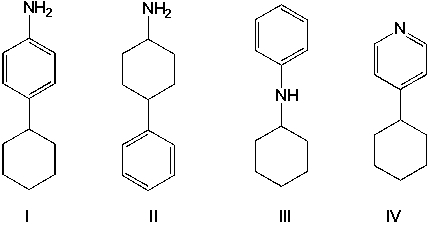
A) II, III, I, IV
B) IV, III, II, I
C) I, III, IV, II
D) III, IV, I, II

A) II, III, I, IV
B) IV, III, II, I
C) I, III, IV, II
D) III, IV, I, II
II, III, I, IV
2
Which compound is the strongest base?

A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV
IV
3
Which is the name for the following compound?
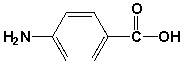
A) 4-carboxy aniline
B) 1-amino-4-carboxybenzene
C) benzylamine carboxylic acid
D) 4-aminobenzoic acid

A) 4-carboxy aniline
B) 1-amino-4-carboxybenzene
C) benzylamine carboxylic acid
D) 4-aminobenzoic acid
4-aminobenzoic acid
4
Which is the IUPAC name for the following structure?

A) N-methyl-tert-butylamine
B) 2,N-methyl-1,1-dimethylethylamine
C) 2,N-dimethyl-2-propanamine
D) tert-butyl methyl amine

A) N-methyl-tert-butylamine
B) 2,N-methyl-1,1-dimethylethylamine
C) 2,N-dimethyl-2-propanamine
D) tert-butyl methyl amine

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
5
Arrange the amines in order of increasing boiling point (lowest first).

A) I, III, II, IV
B) II, IV, I, III
C) IV, II, III, I
D) II, III, IV, I

A) I, III, II, IV
B) II, IV, I, III
C) IV, II, III, I
D) II, III, IV, I

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
6
Hydrocodone is a semi-synthetic opioid, which is an orally active narcotic analgesic (painkiller), often found (together with acetaminophen (N-(4-hydroxyphenyl)acetamide)) in Vicodin and other brands. What is the classification of the amine?

A) tertiary aromatic
B) tertiary aliphatic
C) secondary aliphatic
D) primary aliphatic

A) tertiary aromatic
B) tertiary aliphatic
C) secondary aliphatic
D) primary aliphatic

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
7
Which is the classification of the following compound?

A) 1°
B) 2°
C) 3°
D) 4°

A) 1°
B) 2°
C) 3°
D) 4°

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
8
Arrange the amines in order of increasing solubility in water (least first).

A) II, III, I, IV
B) IV, II, III, I
C) IV, III, II, I
D) I, III, II, IV

A) II, III, I, IV
B) IV, II, III, I
C) IV, III, II, I
D) I, III, II, IV

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
9
Arrange the amines in order of increasing boiling point (lowest first).
Methylamine ethylamine propylamine cyclohexylamine
I II III IV
A) IV, III, II, I
B) I, II, III, IV
C) III, II, I, IV
D) II, I, IV, III
Methylamine ethylamine propylamine cyclohexylamine
I II III IV
A) IV, III, II, I
B) I, II, III, IV
C) III, II, I, IV
D) II, I, IV, III

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
10
Which is the structure for N,N-dimethyl-3-hexanamine?
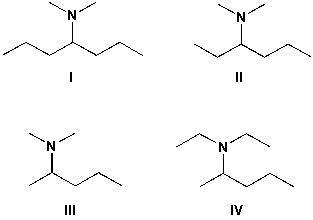
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
11
Which is the correct structure for diphenylamine?

A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
12
Dopamine is a neurotransmitter occurring in a wide variety of animals, including both vertebrates and invertebrates. What is the classification of the amine?

A) primary aromatic
B) primary aliphatic
C) secondary aromatic
D) tertiary aliphatic

A) primary aromatic
B) primary aliphatic
C) secondary aromatic
D) tertiary aliphatic

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
13
Which pairs of names and structures match correctly?

A) I and II and III
B) II and IV and IV
C) II and IV and V
D) I and III and V

A) I and II and III
B) II and IV and IV
C) II and IV and V
D) I and III and V

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
14
Arrange the amines in order of increasing boiling point (lowest first).

A) II, III, I, IV
B) III, I, IV, II
C) IV, II, III, I
D) IV, III, I, II

A) II, III, I, IV
B) III, I, IV, II
C) IV, II, III, I
D) IV, III, I, II

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
15
Arrange the amines in order of increasing solubility in water (least first).

A) III, I, IV, II
B) II, I, III, IV
C) I, III, IV, II
D) IV, II, III, I

A) III, I, IV, II
B) II, I, III, IV
C) I, III, IV, II
D) IV, II, III, I

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
16
Arrange the following in order of increasing strength of the hydrogen bonds (weakest first).
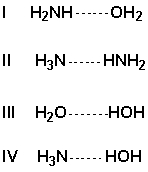
A) I, II, III, IV
B) II, I, IV, III
C) II, IV, I, III
D) I, IV, II, III

A) I, II, III, IV
B) II, I, IV, III
C) II, IV, I, III
D) I, IV, II, III

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
17
Which compound is the strongest base?

A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
18
Procaine is a local anesthetic drug. What is the classification of the amines I and II?

A) I: primary aliphatic; II: primary aromatic
B) I: tertiary aliphatic, II: primary aromatic
C) I: tertiary aliphatic, II: tertiary aromatic
D) I: tertiary aromatic, II: tertiary aliphatic

A) I: primary aliphatic; II: primary aromatic
B) I: tertiary aliphatic, II: primary aromatic
C) I: tertiary aliphatic, II: tertiary aromatic
D) I: tertiary aromatic, II: tertiary aliphatic

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
19
Which is the name for the following compound?
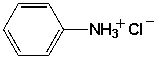
A) aniline hydrochloride
B) phenyltrihydrogenammonium chloride
C) aniline chloride
D) benzylammonium chloride

A) aniline hydrochloride
B) phenyltrihydrogenammonium chloride
C) aniline chloride
D) benzylammonium chloride

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
20
Order the following bases according to increasing basicity (weakest base first):

A) I, III, II, IV
B) III, II, IV, I
C) IV, II, III, I
D) II, IV, I, III

A) I, III, II, IV
B) III, II, IV, I
C) IV, II, III, I
D) II, IV, I, III

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
21
Which structures are secondary amines?

A) I, II
B) II, III
C) III, IV
D) II, IV

A) I, II
B) II, III
C) III, IV
D) II, IV

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
22
The name of the following compound is _______________________________.



Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
23
The name of the following compound is ______________________________.



Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
24
The order of increasing basicity of the following compounds is (1 is the least basic),



Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
25
The starting material needed to complete the following reaction is,



Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
26
The name of the following compound is _________________________________.
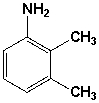


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
27
The major product of the following reaction is,



Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
28
At pH 5.0, the ratio of morpholine and morpholinium ion is 1:1622. Which is the pKb of morpholine?
A) 3.21
B) 5.79
C) 8.21
D) 9.25
A) 3.21
B) 5.79
C) 8.21
D) 9.25

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
29
When the following compounds are dissolved in water at the same concentrations, which one will have the lowest pH (i.e., most acidic)?

A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
30
Which reactions will proceed predominantly to products as written?

A) I, II
B) III, IV
C) II, IV
D) I, III

A) I, II
B) III, IV
C) II, IV
D) I, III

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
31
The following mixture was extracted with 1 M HCl, followed by 1 M NaOH, followed by ether. Which compound is recovered from the acid solution?

A) I
B) II
C) III
D) None of the above

A) I
B) II
C) III
D) None of the above

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
32
The starting material needed to complete the following reaction is,



Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
33
Which is the product of the following reaction?


A) I
B) II
C) III
D) IV


A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
34
The starting material and reactants needed to complete the following reaction are?



Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
35
The order of decreasing basicity of the following amines is (1 is the most basic),



Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
36
Arrange the amines in order of increasing basicity (weakest first).

A) IV, II, III, I
B) II, I, III, IV
C) I, II, III, IV
D) II, I, IV, III

A) IV, II, III, I
B) II, I, III, IV
C) I, II, III, IV
D) II, I, IV, III

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
37
The order of increasing boiling point of the following compounds is (1 is lowest boiling),



Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
38
When guanidine (at left) and imidazole (at right) undergo protonation, which nitrogen atoms are protonated preferentially?

A) I, III
B) II, IV
C) I, IV
D) II, III

A) I, III
B) II, IV
C) I, IV
D) II, III

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
39
Guanidine has a pKb value of 0.4. Which is the ratio of guanidine to its conjugate acid at blood pH of 7.4?
A) 10-6.2:1
B) 10+6.2:1
C) 1:1
D) 2.5:1
A) 10-6.2:1
B) 10+6.2:1
C) 1:1
D) 2.5:1

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
40
Trinitrotoluene (TNT) reacts with large quantities of hydrogen in the presence of a nickel catalyst. Which reaction product is formed?


A) I
B) II
C) III
D) IV


A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
41
The pKa for ethyl ammonium ion is 10.8 (ethyl amine pKb is 3.2).

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
42
The reactants and solvents needed to complete the following reaction are?
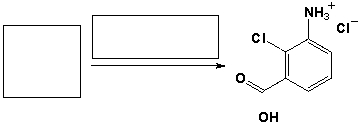


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
43
The starting material and reactants needed to complete the following reaction are?

A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
44
The strongest base in the following group is methylthiocyclohexane.
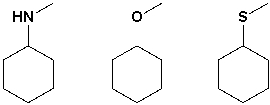


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
45
The strongest base in the following group is aniline.



Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
46
The equilibrium for the reaction of p-nitroaniline and acetic acid lies to the right.



Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
47
Many d-block metals are suitable catalysts for the reduction of aromatic nitro-compounds by molecular hydrogen.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
48
Tertiary amines are generally stronger bases than secondary amines.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
49
The name of the following compound is N-cyclopentylethanamine.



Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
50
Metal catalysts are the more reactive the larger their surface is that is accessible to both, the aromatic nitro-compounds and molecular hydrogen.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
51
Cyclohexanamine is a 2º amine.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
52
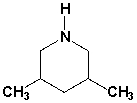
The name of the following compound is 3,5-dimethylpiperidine.


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
53
The reduction of aromatic nitro-compounds using metallic iron requires a basic aqueous reaction medium.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
54
tert-Butyl methyl amine is a 3º amine.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
55
The reduction of aromatic nitro-compounds using molecular hydrogen requires a metal catalyst.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck


