Deck 8: Electronic Structure
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/100
Play
Full screen (f)
Deck 8: Electronic Structure
1
The angular momentum quantum number affects the spatial distribution of the electron in three-dimensional space.
True
2
The electron configuration for Ca (20 electrons) is 1s22s22p63s23p64s2.
True
3
The valence shell electron configuration of S is 3s23p5.
False
4
The element Na has eleven valence electrons.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
5
The 5s subshell is filled before allotting electrons to the 4d subshell.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
6
The electron configuration for Se (38 electrons) is 1s22s22p63s23p64s23d104p64d2.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
7
The valence shell electron configuration of the element K is 4s1

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
8
The first two columns of the periodic table are labeled the s block.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
9
Calcium is an element found in the d block of periodic table.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
10
Frequency is the number of cycles of light that pass a given point in one minute .

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
11
The wavelength of light is the number of cycles of light that pass a given point in one second.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
12
No two electrons in an atom can have the same set of four quantum numbers.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
13
The frequency of light is 1016 s -1 if its wavelength is 10−8 m.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
14
Helium has two electrons. Its electron configuration is 1s11p1.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
15
The spin quantum number of an electron should be between zero and 1.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
16
Frequency is represented by the Greek symbol, ν (Nu).

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
17
A line spectrum can be obtained when the visible portion of the electromagnetic spectrum is passed through a prism.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
18
The wavelength of light is 3 × 10-7 m if its frequency is 1015 s -1.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
19
Electrons within a shell will have the same value of magnetic quantum number.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
20
The energy of light is 2.1 × 10−23 J if its frequency is 3.2 × 1010 s−1.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
21
What is the energy of light if its frequency is 2.69 × 1010 s−1?
A) 3.7 × 10-14 J
B) 9.01 ×1016 J
C) 1.78 × 10-23 J
D) 2.69 × 10-34 J
E) 2.69 × 10-24 J
A) 3.7 × 10-14 J
B) 9.01 ×1016 J
C) 1.78 × 10-23 J
D) 2.69 × 10-34 J
E) 2.69 × 10-24 J

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
22
The _____ quantum number largely determines the energy of an electron.
A) magnetic
B) parallel
C) spin
D) principal
E) angular momentum
A) magnetic
B) parallel
C) spin
D) principal
E) angular momentum

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
23
Quantities that can only have certain specific values are called _____ values.
A) revoked
B) quantized
C) normalized
D) optimized
E) integrated
A) revoked
B) quantized
C) normalized
D) optimized
E) integrated

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
24
Going across a row on the periodic table, left to right, atomic radii decrease.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
25
What is the frequency of light if its wavelength is 5.36 × 10−6 m?
A) 2.12 × 102 s-1
B) 5.60 × 1013 s-1
C) 2.12 × 1014 s-1
D) 1.61 × 103 s-1
E) 1.79 × 10-14 s-1
A) 2.12 × 102 s-1
B) 5.60 × 1013 s-1
C) 2.12 × 1014 s-1
D) 1.61 × 103 s-1
E) 1.79 × 10-14 s-1

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
26
The distance between corresponding points in two adjacent light cycles is called _____.
A) quantum
B) spectral line
C) frequency
D) spectrum
E) wavelength
A) quantum
B) spectral line
C) frequency
D) spectrum
E) wavelength

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
27
Rainbow is an example of a(n) _____ spectrum.
A) absorption
B) line
C) mass
D) continuous
E) power
A) absorption
B) line
C) mass
D) continuous
E) power

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following spectra appear when electricity is passed through a gas and light is emitted and this light is passed though a prism?
A) Continuous spectrum
B) Integrated spectrum
C) Line spectrum
D) Visible spectrum
E) Absorption spectrum
A) Continuous spectrum
B) Integrated spectrum
C) Line spectrum
D) Visible spectrum
E) Absorption spectrum

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
29
Ionization energies increase as you go across the periodic table from left to right.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
30
What is the visible wavelength range in the electromagnetic spectrum?
A) 800 nm to 1200 nm
B) 400 nm to 700 nm
C) 200 nm to 300 nm
D) 50 nm to 500 nm
E) 20 nm to 200 nm
A) 800 nm to 1200 nm
B) 400 nm to 700 nm
C) 200 nm to 300 nm
D) 50 nm to 500 nm
E) 20 nm to 200 nm

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is the quantum number that has a minor effect on the energy of the electron and affects the spatial distribution of the electron in three-dimensional space?
A) parallel
B) principal
C) magnetic
D) spin
E) angular momentum
A) parallel
B) principal
C) magnetic
D) spin
E) angular momentum

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
32
Electron affinity refers to the energy change when a gas-phase atom accepts an electron.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
33
The atomic radius of carbon is greater than that of boron.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following statements is consistent with the theory of quantum mechanics?
A) Electrons do not have specific wavelengths
B) Electrons are collected into groups and subgroups
C) Electrons act as waves rather than particles
D) Electrons are neutrally charged particles
E) Electrons are not randomly distributed around a nucleus
A) Electrons do not have specific wavelengths
B) Electrons are collected into groups and subgroups
C) Electrons act as waves rather than particles
D) Electrons are neutrally charged particles
E) Electrons are not randomly distributed around a nucleus

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is the reason why electrons have quantized energy values?
A) Electrons have high wavelength
B) Electrons emit electromagnetic radiation
C) Electrons have high frequency
D) Electrons have negative charge
E) Electrons exist in specific orbits
A) Electrons have high wavelength
B) Electrons emit electromagnetic radiation
C) Electrons have high frequency
D) Electrons have negative charge
E) Electrons exist in specific orbits

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
36
What is the wavelength of light if its frequency is 1.6 × 1014 s-1?
A) 5.3 × 10−7 m
B) 1.9 × 10-6 m
C) 4.8 × 1022 m
D) 4.8 × 10-8 m
E) 1.5 × 107 m
A) 5.3 × 10−7 m
B) 1.9 × 10-6 m
C) 4.8 × 1022 m
D) 4.8 × 10-8 m
E) 1.5 × 107 m

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
37
The number of cycles of light that pass a given point in one second is the_____ of light.
A) wavelength
B) spectrum
C) spectral line
D) frequency
E) altitude
A) wavelength
B) spectrum
C) spectral line
D) frequency
E) altitude

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
38
Atomic radii decrease as you go down a column of the periodic table.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
39
What is the frequency of light if its wavelength is 813 nm?
A) 3.69 × 1014 s-1
B) 2.71 × 10-14 s-1
C) 2.41 × 102 s-1
D) 8.10 × 1014 s-1
E) 2.71 × 10-15 s-1
A) 3.69 × 1014 s-1
B) 2.71 × 10-14 s-1
C) 2.41 × 102 s-1
D) 8.10 × 1014 s-1
E) 2.71 × 10-15 s-1

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
40
The energy of light is estimated to be 6.56 × 10−21 J. The frequency of this light is _____ s-1.
A) 1.02 × 10-13
B) 4.34 × 100
C) 9.90 × 1013
D) 1.02 × 10-13
E) 9.90 × 1012
A) 1.02 × 10-13
B) 4.34 × 100
C) 9.90 × 1013
D) 1.02 × 10-13
E) 9.90 × 1012

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following magnetic quantum numbers is not possible if the principal quantum number is 4?
A) 5
B) 2
C) 1
D) 0
E) -1
A) 5
B) 2
C) 1
D) 0
E) -1

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following is a highest principal quantum number for the electrons in a calcium atom?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following sets of quantum numbers is shared by electrons in the same orbital?
A) {2, 1, 0, −1/2} and {2, 1, 1, +1/2}
B) {2, 1, 0, +1/2} and {2, 1, 0, −1/2}
C) {3, 1, 1 , −1/2} and {3, 1, −1, +1/2}
D) {3, 2, 0, +1/2} and {3, 2, 1, −1/2}
E) {1, 1, −1, −1/2} and {1, 1, 1, + 1/2}
A) {2, 1, 0, −1/2} and {2, 1, 1, +1/2}
B) {2, 1, 0, +1/2} and {2, 1, 0, −1/2}
C) {3, 1, 1 , −1/2} and {3, 1, −1, +1/2}
D) {3, 2, 0, +1/2} and {3, 2, 1, −1/2}
E) {1, 1, −1, −1/2} and {1, 1, 1, + 1/2}

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
44
How many subshells will have electrons if the atom has 15 electrons?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following refers to the p block in the periodic table?
A) First two rows of the periodic table
B) Right-most six columns of the periodic table
C) First two columns of the periodic table
D) Middle 10 columns of the periodic table
E) The section that is detached from the main body of the table
A) First two rows of the periodic table
B) Right-most six columns of the periodic table
C) First two columns of the periodic table
D) Middle 10 columns of the periodic table
E) The section that is detached from the main body of the table

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
46
Identify the subshell that is expected to remain partially filled for an atom with 55 electrons.
A) 4f
B) 5p
C) 4d
D) 6s
E) 5s
A) 4f
B) 5p
C) 4d
D) 6s
E) 5s

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
47
Each value of _____ quantum number designates a certain orbital.
A) spin
B) angular
C) magnetic
D) parallel
E) principal
A) spin
B) angular
C) magnetic
D) parallel
E) principal

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following elements belong to the p block in the periodic table?
A) Li
B) Na
C) O
D) K
E) H
A) Li
B) Na
C) O
D) K
E) H

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
49
Electrons in the last unfilled subshell of an atom are called _____ electrons.
A) inactive
B) noble
C) kinetic
D) valence
E) dormant
A) inactive
B) noble
C) kinetic
D) valence
E) dormant

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
50
An electron is characterized by the following quantum number values. n = 3; ℓ = 2; mℓ = 1. Which of the following can be the spin quantum number for this electron?
A) 0
B) 1
C) 2
D) −1/2
E) +1/6
A) 0
B) 1
C) 2
D) −1/2
E) +1/6

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following numbers can be the value for angular momentum quantum number if the principal quantum is 1?
A) -1
B) 0
C) 1
D) 2
E) 3
A) -1
B) 0
C) 1
D) 2
E) 3

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
52
The first two columns of the periodic table are labeled the _____.
A) p block
B) d block
C) a block
D) f block
E) s block
A) p block
B) d block
C) a block
D) f block
E) s block

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following set of quantum numbers {n, ℓ, mℓ, ms} is allowed?
A) {2, 1, 0, −1/2}
B) {2, 1, 2, +1/2}
C) {2, 2, 0, −1/2}
D) {2, −1, 0, +1/2}
E) {2, 2, 1, −1/2}
A) {2, 1, 0, −1/2}
B) {2, 1, 2, +1/2}
C) {2, 2, 0, −1/2}
D) {2, −1, 0, +1/2}
E) {2, 2, 1, −1/2}

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
54
What are the valence shell electron configurations of the elements in the first column of the periodic table?
A) np2
B) ns2
C) np1
D) np3
E) ns1
A) np2
B) ns2
C) np1
D) np3
E) ns1

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
55
How many valence electrons are present in a Ni atom with 28 electrons?
A) 1
B) 2
C) 3
D) 5
E) 9
A) 1
B) 2
C) 3
D) 5
E) 9

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following is the correct electronic configuration for an Mn atom with 25 electrons?
A)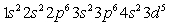
B)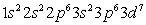
C)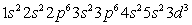
D)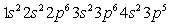
E)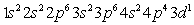
A)
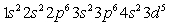
B)
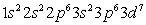
C)
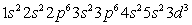
D)
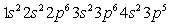
E)
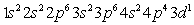

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following is the central idea of the Pauli exclusion principle?
A) Electrons should have equal principal and magnetic quantum numbers.
B) At least three electrons should exist in a subshell.
C) Electrons have negative charge and positive magnetic polarization.
D) Electrons contain all colors of light.
E) No two electrons in an atom can have the same set of four quantum numbers.
A) Electrons should have equal principal and magnetic quantum numbers.
B) At least three electrons should exist in a subshell.
C) Electrons have negative charge and positive magnetic polarization.
D) Electrons contain all colors of light.
E) No two electrons in an atom can have the same set of four quantum numbers.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following elements belongs to the s block in the periodic table?
A) Fe
B) Ne
C) C
D) Na
E) N
A) Fe
B) Ne
C) C
D) Na
E) N

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
59
How many subshells are completely filled with an atom that has 40 electrons?
A) 9
B) 12
C) 8
D) 6
E) 4
A) 9
B) 12
C) 8
D) 6
E) 4

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is an element in the d block of the periodic table?
A) Cl
B) Fe
C) Na
D) K
E) Ne
A) Cl
B) Fe
C) Na
D) K
E) Ne

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
61
Referring to the periodic table, which of the following atoms is likely to have the smallest atomic radius?
A) Sc
B) Cr
C) Co
D) Cu
E) Zn
A) Sc
B) Cr
C) Co
D) Cu
E) Zn

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
62
How would the eight electrons for O be assigned to the n and ℓ quantum numbers? Briefly explain.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
63
Briefly distinguish between continuous spectrum and line spectrum.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
64
Referring to the periodic table, which of the following atoms is likely to have the largest atomic radius?
A) C
B) O
C) B
D) F
E) N
A) C
B) O
C) B
D) F
E) N

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
65
Referring to the periodic table, which of the following atoms is likely to have the highest ionization energy?
A) P
B) S
C) Al
D) Cl
E) Si
A) P
B) S
C) Al
D) Cl
E) Si

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
66
What is electromagnetic spectrum? Which part of electromagnetic spectrum is visible?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
67
_____ is the amount of energy required to remove an electron from an atom in the gas phase.
A) Electron affinity
B) Kinetic energy
C) Nuclear affinity
D) Magnetic energy
E) Ionization energy
A) Electron affinity
B) Kinetic energy
C) Nuclear affinity
D) Magnetic energy
E) Ionization energy

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
68
Explain the relationship between wavelength and frequency of light.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
69
Referring to the periodic table, which of the following atoms is likely to have the highest ionization energy?
A) F
B) Cl
C) Br
D) I
E) At
A) F
B) Cl
C) Br
D) I
E) At

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
70
What is spin quantum number? What are the values that it can have?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
71
What is quantum number? Give a set of quantum numbers that exist.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
72
The inner electrons of an atom are called _____.
A) noble electrons
B) idle electrons
C) redundant electrons
D) core electrons
E) valence electrons
A) noble electrons
B) idle electrons
C) redundant electrons
D) core electrons
E) valence electrons

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
73
Referring to the periodic table, which of the following atoms has the smallest atomic radius?
A) Li
B) Rb
C) Na
D) H
E) K
A) Li
B) Rb
C) Na
D) H
E) K

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
74
What is the abbreviated electron configuration for S, which has 16 electrons?
A) [Ar]4s1
B) [Ar]3p2
C) [Ne] 3s23p4
D) [Ne][Ar]4s2
E) [Ne]3p3
A) [Ar]4s1
B) [Ar]3p2
C) [Ne] 3s23p4
D) [Ne][Ar]4s2
E) [Ne]3p3

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
75
Explain the concepts of wavelength and frequency of light. Draw a wave and show wavelength and frequency.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
76
Predict which of the following atoms will have the highest magnitude of electron affinity.
A) B
B) F
C) O
D) N
E) C
A) B
B) F
C) O
D) N
E) C

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
77
List the four types of quantum numbers.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
78
What are s block elements? Provide an example.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following is the partially filled subshell for an atom that has 46 electrons?
A) 4d8
B) 3d10
C) 4d10
D) 4f1
E) 5s2
A) 4d8
B) 3d10
C) 4d10
D) 4f1
E) 5s2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
80
Briefly explain Pauli's exclusion principle.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck


