Deck 11: Solutions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/80
Play
Full screen (f)
Deck 11: Solutions
1
Solutions have a higher boiling point than pure solvents.
True
2
Solutions exist in all phases of matter.
True
3
Solutions have a lower vapor pressure than the pure solvent.
True
4
The freezing point of a 0.5 m solution of NaCl is water is less than 0°C.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
5
CH3OH is not soluble in water

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
6
Solutions have a higher freezing point than their pure solvents.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
7
A solution is made by mixing 2.00 mol of solute and 15.0 mol of solvent. The mole fraction of the solute is 0.133

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
8
Molarity of a solution is the number of moles of solute divided by the milliliters of the solution.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
9
NaOH will be more soluble in CCl4 than H2O.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
10
The boiling point of a 5.00 m solution of C6H4Cl2 in CCl4 is 101.6°C. Assume that C6H4Cl2 is not volatile and Kb = 4.95°C/m.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
11
The freezing point of a 1.9 m solution of NaCl in H2O is -3.5°C if Kf = 1.86°C/m.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
12
Molality refers to the number of moles of solute per gram of solvent.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following terms refer to the major component of a solution?
A) solute
B) solvent
C) precipitate
D) stream
E) pour out
A) solute
B) solvent
C) precipitate
D) stream
E) pour out

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
14
The van't Hoff factor for CaCO3 is 2.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
15
The van't Hoff factor for CCl4 is 5.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
16
For ionic solutions, the total ion concentration is the sum of the individual ion concentrations.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
17
In both dilution and concentration, the amount of solute stays the same.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
18
The molarity of a solution is 25 M if 5.0 moles of the solute is present in 5.0. liters of the solution.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
19
Osmotic pressure is the tendency of a solution to pass solution through a semipermeable membrane due to concentration differences.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
20
If 25.0 mL of a 1.0 M solution is diluted to 50.0 mL, the concentration becomes 0.50 M.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
21
What mass of CaCl2 is needed to produce 15.0 liters of 1.60 M solution of CaCl2? (Molar mass of CaCl2 = 111 g)
A) 2.40 kg
B) 2.66 kg
C) 1,040 g
D) 24.0 g
E) 2.08 kg
A) 2.40 kg
B) 2.66 kg
C) 1,040 g
D) 24.0 g
E) 2.08 kg

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
22
The process of adding solvent to a solution is called _____.
A) concentration
B) saturation
C) precipitation
D) ionization
E) dilution
A) concentration
B) saturation
C) precipitation
D) ionization
E) dilution

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
23
What is the molarity of a solution if 15 g NaCl is present in 0.50 L of the solution? (Molar mass of NaCl = 58.45 g/mol)
A) 0.26 M
B) 7.5 M
C) 15 M
D) 0.51M
E) 3.0 M
A) 0.26 M
B) 7.5 M
C) 15 M
D) 0.51M
E) 3.0 M

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
24
What mass of solute is present in 2.050 L of 0.6630 M NaOH? (Molar mass of NaOH = 40.00 g/mol)
A) 82.00 g
B) 54.37 g
C) 66.30 g
D) 13.26 g
E) 10.25 g
A) 82.00 g
B) 54.37 g
C) 66.30 g
D) 13.26 g
E) 10.25 g

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
25
The concentration of Cl- ion in a sample of H2O is 21.0 ppth. What mass of Cl- ion is present in 0.900 kg of H2O?
A) 9.45 g
B) 9.45 mg
C) 18.9 mg
D) 21 g
E) 18.9 g
A) 9.45 g
B) 9.45 mg
C) 18.9 mg
D) 21 g
E) 18.9 g

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
26
What is the molarity of a solution made when 46.76 g of NaCl is dissolved to make 500.0 mL of solution? (Molar mass of NaCl = 58.45 g/mol)
A) 2.500 M
B) 0.002500 M
C) 1.600 M
D) 80.00 M
E) 0.001600 M
A) 2.500 M
B) 0.002500 M
C) 1.600 M
D) 80.00 M
E) 0.001600 M

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
27
A solution contains 65.0 g of solvent. How much solute is present in the solution if the mole fraction of the solute is 0.135? (Molar mass of solvent = 18 g/mol; molar mass of solute = 30 g/mol)
A) 0.563 g
B) 4.05 g
C) 3.61 g
D) 53.3 g
E) 16.9 g
A) 0.563 g
B) 4.05 g
C) 3.61 g
D) 53.3 g
E) 16.9 g

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
28
How many moles of a solute are present in 200.00 mL of a 1.50 M HCl solution?
A) 0.300 mol
B) 3.00 mol
C) 3.00 × 102 mol
D) 75.0 mol
E) 7.50 mol
A) 0.300 mol
B) 3.00 mol
C) 3.00 × 102 mol
D) 75.0 mol
E) 7.50 mol

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
29
The number of moles of solute divided by the number of liters of solution is _____.
A) concentration
B) molar volume
C) dilution
D) volume deliberation
E) molarity
A) concentration
B) molar volume
C) dilution
D) volume deliberation
E) molarity

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
30
Identify the solvent in which C2H5OH will be most soluble.
A) CH4
B) CCl4
C) C6H6
D) H2O
E) CO2
A) CH4
B) CCl4
C) C6H6
D) H2O
E) CO2

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
31
_____ refers to a property of solutions related to the fraction that the solute particles occupy in the solution, not their identity.
A) Dissolutive property
B) Static property
C) Dynamic property
D) Kinetic property
E) Colligative property
A) Dissolutive property
B) Static property
C) Dynamic property
D) Kinetic property
E) Colligative property

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
32
How much NaCl is present in 15 L of a 1.5 M solution of NaCl? (Molar mass of NaCl = 58.5 g/mol)
A) 1.32 kg
B) 22.5 kg
C) 2.25 kg
D) 315 g
E) 565 g
A) 1.32 kg
B) 22.5 kg
C) 2.25 kg
D) 315 g
E) 565 g

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
33
How many moles of HCl are present in 1.20 L of a 0.850 M solution?
A) 2.05 mol
B) 1.02 mol
C) 0.980 mol
D) 0.708 mol
E) 1.68 mol
A) 2.05 mol
B) 1.02 mol
C) 0.980 mol
D) 0.708 mol
E) 1.68 mol

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
34
If there is 9.00 g of Ag present in 190 g of solution, what is the Ag concentration in parts per thousand?
A) 21.0 ppth
B) 1.70 ppth
C) 47 ppth
D) 47.4 ppth
E) 17.0 ppth
A) 21.0 ppth
B) 1.70 ppth
C) 47 ppth
D) 47.4 ppth
E) 17.0 ppth

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
35
A solution is made by dissolving 15.00 g of NaCl in 500.0 g of H2O. What is the mole fraction of NaCl in the solution?
A) 0.0089
B) 0.2566
C) 0.0092
D) 0.1156
E) 0.2778
A) 0.0089
B) 0.2566
C) 0.0092
D) 0.1156
E) 0.2778

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
36
A solution is made by mixing 25.0 g of NaOH in H2O. What is the amount of H2O in the solution? (Mole fraction of solute = 0.090; molar mass of NaOH = 40.0 g/mol; molar mass of H2O = 18.0 g/mol)
A) 113.8 g
B) 56.25 g
C) 256.5 g
D) 6.32 g
E) 125.6 g
A) 113.8 g
B) 56.25 g
C) 256.5 g
D) 6.32 g
E) 125.6 g

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
37
Only a certain amount of solute can be dissolved in a given amount of solvent. This maximum amount is called the _____ of the solute.
A) solubility
B) reactivity
C) dilution
D) hydraulicity
E) acidity
A) solubility
B) reactivity
C) dilution
D) hydraulicity
E) acidity

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
38
A concentrated solution refers to a solution that has _____.
A) very low density
B) very high viscosity
C) large amounts of solute
D) large amounts of solvent
E) very high density
A) very low density
B) very high viscosity
C) large amounts of solute
D) large amounts of solvent
E) very high density

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
39
The molarity of a solution made by dissolving 2.00 moles of HCl in water is 2.65 M. What is the volume of the solution?
A) 2.65 L
B) 755 mL
C) 265 mL
D) 0.53 L
E) 53 mL
A) 2.65 L
B) 755 mL
C) 265 mL
D) 0.53 L
E) 53 mL

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
40
The concentration of Br- ion in a sample of H2O is 10 ppm. What mass of Br- ion is present in 300.0 mL of H2O, which has a density of 1.02 g/mL?
A) 10 mg
B) 6.00 mg
C) 3.00 mg
D) 3.06 mg
E) 4.52 mg
A) 10 mg
B) 6.00 mg
C) 3.00 mg
D) 3.06 mg
E) 4.52 mg

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
41
What is dilution and concentration?

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
42
A solution is made by mixing 6.000 g of C10H8 in 90.00 g of C6H6. If the vapor pressure of pure C6H6 is 39.30 torr, what is the vapor pressure of the solution? (Molar masses: C10H8 = 128.0 g/mol; C6H6 = 78.00 g/mol)
A) 35.64 torr
B) 1.201 torr
C) 1.843 torr
D) 37.76 torr
E) 0.9609 torr
A) 35.64 torr
B) 1.201 torr
C) 1.843 torr
D) 37.76 torr
E) 0.9609 torr

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
43
You are given 15 liters of a solution of NaCl in water. You estimate that the given solution has a molarity of 0.50 M. How do you increase the molarity of this solution to 1.0 M?

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
44
What is the freezing point of a 10.0 m solution of NaOH in water? (Kf for water = 1.86 °C/m)?
A) -18.6°C
B) -3.20°C
C) -11.6°C
D) -37.2°C
E) -9.25°C
A) -18.6°C
B) -3.20°C
C) -11.6°C
D) -37.2°C
E) -9.25°C

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
45
What is the ideal van't Hoff factor for LiNO3?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
46
What is the freezing point of a 1.8 m solution of CBr4 in C6H6? (Kf for C6H6 = 4.90°C/m; Freezing point of C6H6 = 5.51°C)
A) -6.90 °C
B) 2.30 °C
C) -3.31 °C
D) 0.212 °C
E) 1.20 °C
A) -6.90 °C
B) 2.30 °C
C) -3.31 °C
D) 0.212 °C
E) 1.20 °C

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
47
How do you determine the solute and solvent in a solution? Explain with an example.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
48
The osmotic pressure of blood is 4.83 atm at 47.0°C. If blood were considered a solution of NaCl, what is the molal concentration of NaCl in blood? Assume an ideal van't Hoff factor.
A) 0.345 m
B) 0.690 m
C) 0.920 m
D) 0.460 m
E) 0.230 m
A) 0.345 m
B) 0.690 m
C) 0.920 m
D) 0.460 m
E) 0.230 m

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
49
What is molarity? Explain with an example.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
50
Determine the freezing point of a 2.33 m solution of PCl5 in H2O. (Kf = 1.86°C/m)
A) -8.66°C
B) 8.66°C
C) -2.10°C
D) -4.33°C
E) 1.20°C
A) -8.66°C
B) 8.66°C
C) -2.10°C
D) -4.33°C
E) 1.20°C

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
51
The osmotic pressure of a 0.0750 M KCl solution at 25.0°C is 3.28 atm. What is the true van't Hoff factor of this ionic compound?
A) 3.28
B) 0.0750
C) 0.250
D) 3.48
E) 1.79
A) 3.28
B) 0.0750
C) 0.250
D) 3.48
E) 1.79

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
52
Explain the concentration units parts per thousand (ppth), parts per million (ppm), and parts per billion (ppb).

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
53
The mole fraction of the solute in a solution is 0.1590. If the vapor pressure of the solvent in its pure state is 56.30 torr, what is the vapor pressure of the solution?
A) 56.45 torr
B) 47.35 torr
C) 17.90 torr
D) 49.95 torr
E) 39.55 torr
A) 56.45 torr
B) 47.35 torr
C) 17.90 torr
D) 49.95 torr
E) 39.55 torr

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
54
What is the osmotic pressure of a 2.726 M solution of C6H12O6 at 47.00°C?
A) 98.63 atm
B) 71.57 atm
C) 840.32 atm
D) 10.13 atm
E) 4.656 atm
A) 98.63 atm
B) 71.57 atm
C) 840.32 atm
D) 10.13 atm
E) 4.656 atm

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
55
What is the boiling point of a 5.60 m solution of C6H4Cl2 in CCl4? Assume that C6H4Cl2 is not volatile. (Kb = 4.95°C/m for CCl4; TBP = 76.8°C)
A) 105°C
B) 101°C
C) 89.8°C
D) 91.2°C
E) 123°C
A) 105°C
B) 101°C
C) 89.8°C
D) 91.2°C
E) 123°C

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
56
How many liters of a 0.5 M solution of CaCl2 will contain 10 moles of CaCl2? Explain your calculations.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
57
The boiling point of a solution of NaOH in H2O is 100.84°C. What is the molality of the solution? Assume that NaOH is not volatile. (Kb = 0.51200°C/m for H2O4; TBP = 100.00°C).
A) 1.112 m
B) 1.857 m
C) 1.641m
D) 0.9568 m
E) 0.8205 m
A) 1.112 m
B) 1.857 m
C) 1.641m
D) 0.9568 m
E) 0.8205 m

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
58
Explain the concept of molality.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
59
Explain the concept of concentration in solutions.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
60
What is the van't Hoff factor for Fe(NO3)3?
A) 11
B) 5
C) 2
D) 3
E) 4
A) 11
B) 5
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
61
_____ = 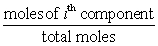
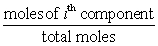

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
62
What are colligative properties?

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
63
The ideal van't Hoff Factor for FeCl3 is _____.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
64
_____ is described by Psoln = χsolvP*solv.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
65
A 10.0 L of a 0.055 M solution are diluted to 15.8 L. The final concentration is _____ M.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
66
_____ is defined as the number of moles of solute per kilogram of solvent.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
67
_____ is the boiling point of a 6.200 m solution of NaCl in H2O. Assume that NaCl is not volatile (Kb for water = 0.5120 °C/m).

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
68
Explain Raoult's law.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
69
Explain the concept of mole fraction with an example.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
70
Explain freezing point depression.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
71
What mass of solute is present in 1 L of 1.60 M solution of NaCl? Explain the calculations.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
72
_____ g solute is present in 15 L of 0.20 M NaOH. (Molar mass of NaOH = 40.0 g/mol)

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
73
The minor component of a solution is called the _____.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
74
A solution that has a lot of solute is called a(n) _____ solution.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
75
The molarity is 3.50 M when _____ mol of NaCl is found in 0.500 L of solution.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
76
What is the reason why you should not drink seawater if you're stranded in a lifeboat on an ocean?

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
77
_____ liters of 0.005 M NaOH are needed to obtain 1.00 mol of NaOH.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
78
Explain the concept of boiling point elevation.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
79
_____ is a property of solutions related to the fraction that the solute particles occupy in the solution, not their identity.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
80
_____ is the addition of solvent, which decreases the concentration of the solute in the solution.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck


