Deck 14: Aldehydes and Ketones
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/44
Play
Full screen (f)
Deck 14: Aldehydes and Ketones
1
The common name for 2-butanone, a readily available solvent, is
A)
B) butyl ketone.
C) methyl acetone.
D) methyl ethyl ketone.
E)butyl ether.
A)

B) butyl ketone.
C) methyl acetone.
D) methyl ethyl ketone.
E)butyl ether.
methyl ethyl ketone.
2
How many lone pairs of electrons does the oxygen in a carbonyl group have?
A)None, they're all bonded.
B)one
C)two
D)three
E)four
A)None, they're all bonded.
B)one
C)two
D)three
E)four
two
3
Which of the following pairs of compounds are isomers?
A)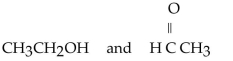
B)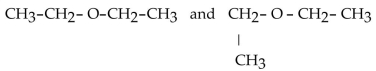
C)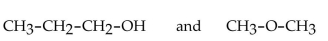
D)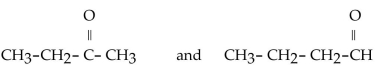
E)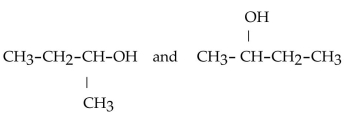
A)
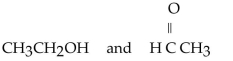
B)
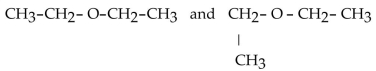
C)
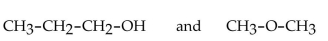
D)
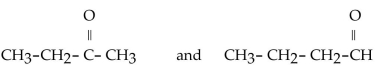
E)
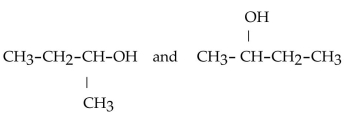
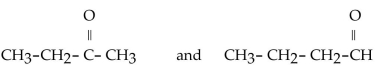
4
What is the IUPAC name for this compound? 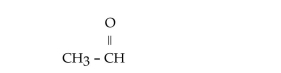
A)methyl aldehyde
B)methanal
C)ethanal
D)1-ethanaldehyde
E)1-ethanone
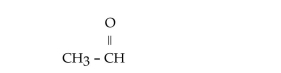
A)methyl aldehyde
B)methanal
C)ethanal
D)1-ethanaldehyde
E)1-ethanone

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
5
What is the IUPAC name for this compound? 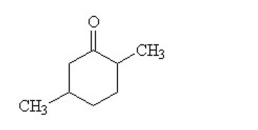
A)2,5-dimethylcyclohexanone
B)1,4-dimethyl-2-cyclohexanone
C)methylcyclohexanone
D)1,4-dimethyl-3-cyclohexanone
E)cyclohexyl methyl ketone

A)2,5-dimethylcyclohexanone
B)1,4-dimethyl-2-cyclohexanone
C)methylcyclohexanone
D)1,4-dimethyl-3-cyclohexanone
E)cyclohexyl methyl ketone

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
6
Which one of the following compounds is an aldehyde?
A)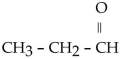
B)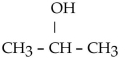
C)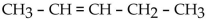
D)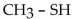
E) CH3-CH2-O-CH2-CH3
A)
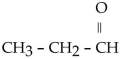
B)
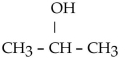
C)
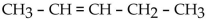
D)
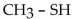
E) CH3-CH2-O-CH2-CH3

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
7
In the IUPAC naming system, an aldehyde is named by replacing the -e of the name of the corresponding alkane with
A)al.
B)one.
C)ene.
D)yne.
E)ol.
A)al.
B)one.
C)ene.
D)yne.
E)ol.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
8
Acetone can be produced by the body when a person is
A)recovering from surgery.
B)sleeping.
C)exercising.
D)dieting with high protein diets.
E)ill with a flu.
A)recovering from surgery.
B)sleeping.
C)exercising.
D)dieting with high protein diets.
E)ill with a flu.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
9
In all aldehydes except formaldehyde, how many hydrogen atoms are bonded to the carbonyl group?
A)one
B)two
C)three
D)four
A)one
B)two
C)three
D)four

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
10
The carbonyl group consists of
A)a carbon singly bonded to an oxygen and the oxygen singly bonded to another carbon.
B)a carbon singly bonded to an oxygen and the oxygen bonded to a hydrogen.
C)a carbon triply bonded to an oxygen.
D)a carbon double bonded to an oxygen.
E)a carbon singly bonded to an oxygen.
A)a carbon singly bonded to an oxygen and the oxygen singly bonded to another carbon.
B)a carbon singly bonded to an oxygen and the oxygen bonded to a hydrogen.
C)a carbon triply bonded to an oxygen.
D)a carbon double bonded to an oxygen.
E)a carbon singly bonded to an oxygen.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the folowing compounds contains a ketone functional group?
A)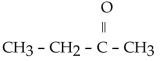
B)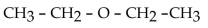
C)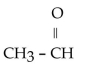
D)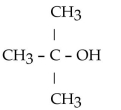
E)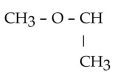
A)
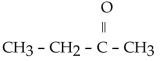
B)
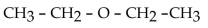
C)
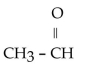
D)

E)
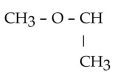

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
12
In the IUPAC naming system, a ketone is named by replacing the -e in the corresponding alkane name with
A)al.
B)ol.
C)yne.
D)one.
E)ene.
A)al.
B)ol.
C)yne.
D)one.
E)ene.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
13
Three functional groups found in this compound are 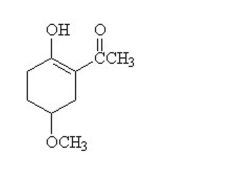
A)alcohol, aldehyde, and ether.
B)alcohol, ether, and ketone.
C)aldehyde, ether, and carboxylic acid.
D)alcohol, aromatic, and ether.
E)cycloalkene, alcohol, and carboxylic acid.

A)alcohol, aldehyde, and ether.
B)alcohol, ether, and ketone.
C)aldehyde, ether, and carboxylic acid.
D)alcohol, aromatic, and ether.
E)cycloalkene, alcohol, and carboxylic acid.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
14
The oxygen atom in a carbonyl group is ________ the carbon atom.
A)more electropositive than
B)more soluble than
C)more electronegative than
D)identical in electronegativity to
E)less electronegative than
A)more electropositive than
B)more soluble than
C)more electronegative than
D)identical in electronegativity to
E)less electronegative than

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
15
Formaldehyde is used industrially to make
A)carpeting.
B)polymers.
C)insulating materials.
D)All of the above.
E)None of the above.
A)carpeting.
B)polymers.
C)insulating materials.
D)All of the above.
E)None of the above.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
16
How many hydrogen atoms are bonded to the carbonyl group in a ketone?
A)none
B)one
C)two
D)three
E)four
A)none
B)one
C)two
D)three
E)four

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
17
How many carbonyl-containing isomers does the formula 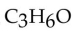 have?
have?
A)two
B)five
C)three
D)eight
E)seven
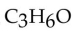 have?
have?A)two
B)five
C)three
D)eight
E)seven

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
18
What is the name of this compound? 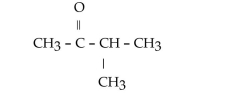
A)2-pentanone
B)2-methyl-3-butanone
C)3-methyl-2-butanone
D)methyl propyl ketone
E)2-methyl-3-ketone butane

A)2-pentanone
B)2-methyl-3-butanone
C)3-methyl-2-butanone
D)methyl propyl ketone
E)2-methyl-3-ketone butane

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
19
The compound 2-propanone is also known as
A) propylone.
B)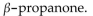
C) 2-propanone.
D) acetone.
E) dimethyl ketone.
A) propylone.
B)
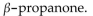
C) 2-propanone.
D) acetone.
E) dimethyl ketone.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
20
What are the bond angles in a typical carbonyl group?
A)
B)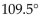
C)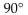
D)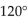
E)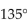
A)

B)
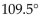
C)
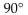
D)
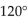
E)
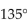

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
21
Aldehydes and ketones may be reduced to
A)alcohols.
B)acids.
C)alkanes.
D)esters.
E)ethers.
A)alcohols.
B)acids.
C)alkanes.
D)esters.
E)ethers.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
22
The flavoring agent found in vanilla is
A)a hydrocarbon.
B)a ketone.
C)an aldehyde.
D)an ester.
E)a thiol.
A)a hydrocarbon.
B)a ketone.
C)an aldehyde.
D)an ester.
E)a thiol.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the compounds would give a positive Tollens' test?
A)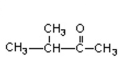
B)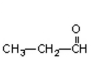
C)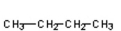
D)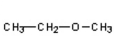
E)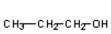
A)

B)

C)
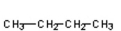
D)
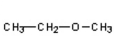
E)
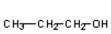

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
24
What is the IUPAC name of this compound?
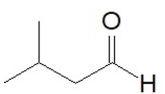
A)3-methylbutanal
B)2-methylbutanone
C)3-methylbutanone
D)pentanone
E)2-methyl-1-butanal

A)3-methylbutanal
B)2-methylbutanone
C)3-methylbutanone
D)pentanone
E)2-methyl-1-butanal

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
25
The Tollens' test may be used to distinguish
A)alcohols from alkenes.
B)ketones from alcohols.
C)acids from amines.
D)esters from acids.
E)aldehydes from ketones.
A)alcohols from alkenes.
B)ketones from alcohols.
C)acids from amines.
D)esters from acids.
E)aldehydes from ketones.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
26
How many moles of an alcohol are needed to react with 1 mole of an aldehyde to form a hemiacetal?
A)3.5 moles
B)3 moles
C)1.5 moles
D)2 moles
E)1 moles
A)3.5 moles
B)3 moles
C)1.5 moles
D)2 moles
E)1 moles

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
27
What is the name of this compound? 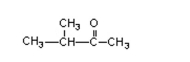
A)3-methylbutanone
B)2-methyl-3-butanone
C)2-methylbutanone
D)pentanone
E)3-methyl-2-butanone
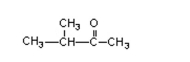
A)3-methylbutanone
B)2-methyl-3-butanone
C)2-methylbutanone
D)pentanone
E)3-methyl-2-butanone

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
28
Formalin is
A)a plastic.
B)another name for formaldehyde.
C)an excellent solvent.
D)a polymer.
E)a 40% aqueous solution of formaldehyde.
A)a plastic.
B)another name for formaldehyde.
C)an excellent solvent.
D)a polymer.
E)a 40% aqueous solution of formaldehyde.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
29
Aldehydes have higher boiling points than alkanes of similar molar mass because of
A)dipole-dipole interactions.
B)ionic bonding.
C)oxygen bonding.
D)covalent bonding.
E)hydrogen bonding.
A)dipole-dipole interactions.
B)ionic bonding.
C)oxygen bonding.
D)covalent bonding.
E)hydrogen bonding.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following ketones is the most solubale in water?
A)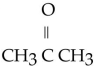
B)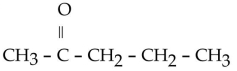
C)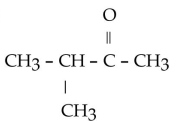
D)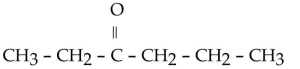
E)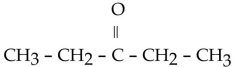
A)
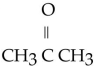
B)
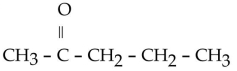
C)

D)
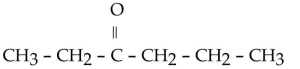
E)
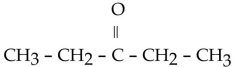

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
31
An acetal is formed from two molecules of an alcohol and a(n)
A)ester.
B)ether.
C)carboxylic acid.
D)aldehyde.
E)alkyl ether.
A)ester.
B)ether.
C)carboxylic acid.
D)aldehyde.
E)alkyl ether.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following would NOT be water soluble?
A)acetone
B)propanal
C)2-butanone
D)formaldehyde
E)3-heptanone
A)acetone
B)propanal
C)2-butanone
D)formaldehyde
E)3-heptanone

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
33
What is the common name of this compound? 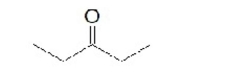
A)acetone
B)3-pentanone
C)ethyl methyl ketone
D)dimethyl ketone
E)diethyl ketone

A)acetone
B)3-pentanone
C)ethyl methyl ketone
D)dimethyl ketone
E)diethyl ketone

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
34
Low-molecular-weight ketones are soluble in water. What is the shortest length of the carbon chain where insolubility becomes important?
A)one
B)two
C)four
D)five
E)eight
A)one
B)two
C)four
D)five
E)eight

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
35
The Benedict's test may be used to distinguish
A)aldehydes from ketones.
B)ketones from alcohols.
C)acids from amines.
D)alcohols from alkenes.
E)esters from acids.
A)aldehydes from ketones.
B)ketones from alcohols.
C)acids from amines.
D)alcohols from alkenes.
E)esters from acids.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
36
The increased boiling point of ketones compared to alkanes and ethers of similar molar mass is due to
A)resonance.
B)a bent chain structure.
C)hydrogen bonding.
D)ionic interactions.
E)dipole-dipole interactions.
A)resonance.
B)a bent chain structure.
C)hydrogen bonding.
D)ionic interactions.
E)dipole-dipole interactions.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
37
The hydrogenation of 2-methylpropanal yields
A)2-methyl-2-propanol.
B)2-methyl-3-propanol.
C)1-butanol.
D)2-methylpropanoic acid.
E)2-methyl-1-propanol.
A)2-methyl-2-propanol.
B)2-methyl-3-propanol.
C)1-butanol.
D)2-methylpropanoic acid.
E)2-methyl-1-propanol.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
38
The reduction of 3-pentanone with hydrogen in the presence of a nickel catalyst will yield
A)pentanaldehyde.
B)3-pentanol.
C)diethyl alcohol.
D)pentane.
E)2-pentene.
A)pentanaldehyde.
B)3-pentanol.
C)diethyl alcohol.
D)pentane.
E)2-pentene.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
39
Acetone is a ketone commonly used as a
A)solvent.
B)preservative.
C)fuel.
D)drain cleaner.
E)flavoring agent.
A)solvent.
B)preservative.
C)fuel.
D)drain cleaner.
E)flavoring agent.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
40
The addition of hydrogen to an organic compound or the loss of oxygen is called
A)halogenation.
B)oxidation.
C)dehydration.
D)reduction.
E)hydration.
A)halogenation.
B)oxidation.
C)dehydration.
D)reduction.
E)hydration.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
41
The product of adding two molecules of an alcohol to one molecule of an aldehyde in the presence of acid is a(n)
A)hemiacetal.
B)ether.
C)hemiether.
D)acetal.
E)hydroxyl group.
A)hemiacetal.
B)ether.
C)hemiether.
D)acetal.
E)hydroxyl group.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
42
How do sugars form cyclic hemiacetals?
A)Functional groups within molecule of sugar react with each other.
B)Two molecules of a sugar react with one another.
C)A molecule of sugar reacts with an added aldehyde.
D)A sugar molecule decomposes.
E)A molecule of sugar reacts with an added alcohol.
A)Functional groups within molecule of sugar react with each other.
B)Two molecules of a sugar react with one another.
C)A molecule of sugar reacts with an added aldehyde.
D)A sugar molecule decomposes.
E)A molecule of sugar reacts with an added alcohol.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
43
Which of these compounds is the hemiacetal that forms when ethanol reacts with propanal?
A)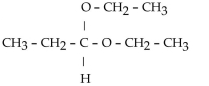
B)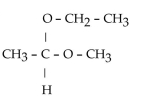
C)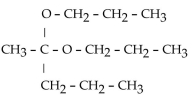
D)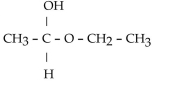
E)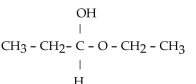
A)
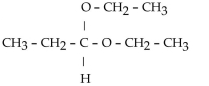
B)
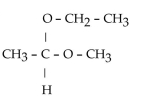
C)
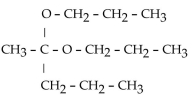
D)
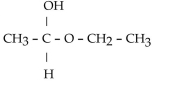
E)
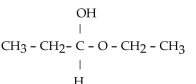

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
44
What is the acetal formed when propanone reacts with two molecules of methanol?
A)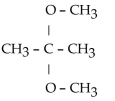
B)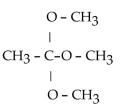
C)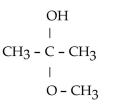
D)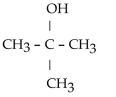
E)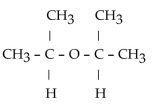
A)
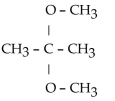
B)
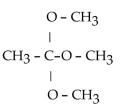
C)

D)

E)
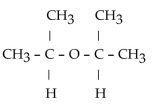

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck


