Deck 4: Atoms and Elements
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/83
Play
Full screen (f)
Deck 4: Atoms and Elements
1
Which of the following elements is a nonmetal?
A)calcium
B)sodium
C)iron
D)nitrogen
E)silver
A)calcium
B)sodium
C)iron
D)nitrogen
E)silver
nitrogen
2
Select the correct symbol for the element.
silver
A)AG
B)Au
C)Ag
D)Si
E)S
silver
A)AG
B)Au
C)Ag
D)Si
E)S
Ag
3
Which of the following elements is a metal?
A)argon
B)fluorine
C)strontium
D)phosphorus
E)nitrogen
A)argon
B)fluorine
C)strontium
D)phosphorus
E)nitrogen
strontium
4
Ca is the symbol for
A)cadmium.
B)copper.
C)cobalt.
D)calcium.
E)carbon.
A)cadmium.
B)copper.
C)cobalt.
D)calcium.
E)carbon.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
5
What is the symbol of the element in Period 4 and Group 2 A (2)?
A)Mg
B)Be
C)Si
D)C
E)Ca
A)Mg
B)Be
C)Si
D)C
E)Ca

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
6
What is the symbol of the element in Group 4A (14)and Period 2?
A)Ca
B)C
C)Si
D)Be
E)Mg
A)Ca
B)C
C)Si
D)Be
E)Mg

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is a characteristic of the modern periodic table?
A)A period is a column on the periodic table.
B)The B groups contain the representative elements.
C)The elements in each group have similar chemical properties.
D)The A groups contain the transition elements.
E)A group is a horizontal row on the periodic table.
A)A period is a column on the periodic table.
B)The B groups contain the representative elements.
C)The elements in each group have similar chemical properties.
D)The A groups contain the transition elements.
E)A group is a horizontal row on the periodic table.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
8
Au is the symbol for
A)argon.
B)sodium.
C)silver.
D)aluminum.
E)gold.
A)argon.
B)sodium.
C)silver.
D)aluminum.
E)gold.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
9
Select the correct symbol for the element.
sodium
A)Sm
B)So
C)Na
D)No
E)Au
sodium
A)Sm
B)So
C)Na
D)No
E)Au

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
10
Select the correct symbol for the element.
potassium
A)Po
B)Pt
C)P
D)K
E)Ko
potassium
A)Po
B)Pt
C)P
D)K
E)Ko

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
11
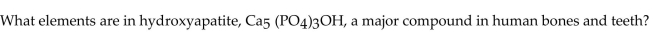
A)carbon, phosphorus, oxygen, helium
B)carbon, potassium, oxygen, helium
C)calcium, phosphorus, oxygen, hydrogen
D)carbon, potassium, oxygen, hydrogen
E)calcium, phosphorus, oxygen, helium

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is a characteristic of nonmetals?
A)good conductors of heat
B)shiny
C)good conductors of electricity
D)malleable
E)low melting points
A)good conductors of heat
B)shiny
C)good conductors of electricity
D)malleable
E)low melting points

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
13
Select the correct symbol for the element.
iron
A)Ir
B)In
C)Fs
D)Fe
E)FE
iron
A)Ir
B)In
C)Fs
D)Fe
E)FE

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following elements is a noble gas?
A)chlorine
B)argon
C)oxygen
D)nitrogen
E)bromine
A)chlorine
B)argon
C)oxygen
D)nitrogen
E)bromine

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
15
Select the correct symbol for the element.
aluminum
A)Au
B)Ag
C)Am
D)Al
E)Sn
aluminum
A)Au
B)Ag
C)Am
D)Al
E)Sn

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
16
Which element would have physical and chemical properties similar to chlorine?
A)Ar
B)O
C)Br
D)S
E)P
A)Ar
B)O
C)Br
D)S
E)P

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
17
The primary substances of which all other things are composed are
A)protons.
B)compounds.
C)electrons.
D)molecules.
E)elements.
A)protons.
B)compounds.
C)electrons.
D)molecules.
E)elements.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
18
Select the correct symbol for the element.
mercury
A)Au
B)Hg
C)Ag
D)Mc
E)Pb
mercury
A)Au
B)Hg
C)Ag
D)Mc
E)Pb

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following properties is NOT a characteristic of the Group 1A (1)elements (alkali metals)?
A)They are shiny.
B)They react vigorously with water.
C)They are good conductors of heat.
D)They are good conductors of electricity.
E)Most of them are liquids at room temperature.
A)They are shiny.
B)They react vigorously with water.
C)They are good conductors of heat.
D)They are good conductors of electricity.
E)Most of them are liquids at room temperature.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
20
The Group 8A (18)elements
A)melt at high temperatures.
B)are unreactive and are rarely found in combination with other elements.
C)are good conductors of electricity.
D)are liquids at room temperature.
E)react vigorously with water.
A)melt at high temperatures.
B)are unreactive and are rarely found in combination with other elements.
C)are good conductors of electricity.
D)are liquids at room temperature.
E)react vigorously with water.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
21
Consider a neutral atom with 30 protons and 34 neutrons. The atomic number of the element is
A)30.
B)32.
C)34.
D)64.
E)94.
A)30.
B)32.
C)34.
D)64.
E)94.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following gives the correct numbers of protons, neutrons, and electrons, respectively, in a neutral atom of 11850 Sn?
A)68 protons, 68 neutrons, 50 electrons
B)118 protons, 50 neutrons, 118 electrons
C)118 protons, 118 neutrons, 50 electrons
D)50 protons, 50 neutrons, 50 electrons
E)50 protons, 68 neutrons, 50 electrons
A)68 protons, 68 neutrons, 50 electrons
B)118 protons, 50 neutrons, 118 electrons
C)118 protons, 118 neutrons, 50 electrons
D)50 protons, 50 neutrons, 50 electrons
E)50 protons, 68 neutrons, 50 electrons

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
23
Consider a neutral atom with 30 protons and 34 neutrons. The number of electrons in this atom is
A)30.
B)32.
C)34.
D)64.
E)94.
A)30.
B)32.
C)34.
D)64.
E)94.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
24
In an atom, the nucleus contains
A)only protons.
B)only neutrons.
C)all the protons and electrons.
D)all the protons and neutrons.
E)an equal number of protons and electrons.
A)only protons.
B)only neutrons.
C)all the protons and electrons.
D)all the protons and neutrons.
E)an equal number of protons and electrons.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
25
The mass number of an atom can be calculated from
A)the number of neutrons.
B)the number of electrons.
C)the number of electrons plus protons.
D)the number of protons plus neutrons.
E)the number of protons.
A)the number of neutrons.
B)the number of electrons.
C)the number of electrons plus protons.
D)the number of protons plus neutrons.
E)the number of protons.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
26
Semiconductors are located in the periodic table on (or in)the
A)line dividing metals from nonmetals in the table.
B)left side of the table.
C)first period of the table.
D)last period of the table.
E)right side of the table.
A)line dividing metals from nonmetals in the table.
B)left side of the table.
C)first period of the table.
D)last period of the table.
E)right side of the table.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
27
The number of neutrons in an atom is equal to
A)the mass number + the atomic number.
B)the atomic number.
C)the number of protons.
D)the mass number.
E)the mass number - the atomic number.
A)the mass number + the atomic number.
B)the atomic number.
C)the number of protons.
D)the mass number.
E)the mass number - the atomic number.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
28
The smallest particle of an element that retains the characteristics of the element is a(n)
A)neutron.
B)atom.
C)nucleus.
D)electron.
E)proton.
A)neutron.
B)atom.
C)nucleus.
D)electron.
E)proton.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
29
Identify the noble gas in the following list.
A)gold
B)chlorine
C)helium
D)oxygen
E)nitrogen
A)gold
B)chlorine
C)helium
D)oxygen
E)nitrogen

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
30
The atomic number of an atom is equal to the number of
A)neutrons.
B)electrons plus protons.
C)neutrons plus protons.
D)protons.
E)nuclei.
A)neutrons.
B)electrons plus protons.
C)neutrons plus protons.
D)protons.
E)nuclei.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
31
Consider a neutral atom with 30 protons and 34 neutrons. The mass number for this atom is
A)30.
B)32.
C)34.
D)64.
E)94.
A)30.
B)32.
C)34.
D)64.
E)94.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following descriptions of a subatomic particle is correct?
A)A proton has a positive charge and a mass of approximately 1 amu.
B)A proton has a positive charge and a negligible mass.
C)An electron has a negative charge and a mass of approximately 1 amu.
D)A neutron has a positive charge and a mass of approximately 1 amu.
E)A neutron has no charge and its mass is negligible.
A)A proton has a positive charge and a mass of approximately 1 amu.
B)A proton has a positive charge and a negligible mass.
C)An electron has a negative charge and a mass of approximately 1 amu.
D)A neutron has a positive charge and a mass of approximately 1 amu.
E)A neutron has no charge and its mass is negligible.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
33
The elements lithium, sodium, and potassium
A)have the same mass number.
B)have the same number of neutrons.
C)are isotopes of each other.
D)are in the same group.
E)are in the same period of elements.
A)have the same mass number.
B)have the same number of neutrons.
C)are isotopes of each other.
D)are in the same group.
E)are in the same period of elements.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
34
Consider an isotope of sodium with a mass number of 25. The number of neutrons in this isotope of sodium is
A)32.
B)11.
C)16.
D)25.
E)14.
A)32.
B)11.
C)16.
D)25.
E)14.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
35
The elements sodium, magnesium, and silicon
A)are in the same period of elements.
B)have the same number of neutrons.
C)have the same mass number.
D)are isotopes of each other.
E)are in the same group.
A)are in the same period of elements.
B)have the same number of neutrons.
C)have the same mass number.
D)are isotopes of each other.
E)are in the same group.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
36
Identify the metalloid in the following list.
A)sulfur
B)copper
C)silver
D)fluorine
E)germanium
A)sulfur
B)copper
C)silver
D)fluorine
E)germanium

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
37
What is the mass number of an atom of potassium that has 20 neutrons?
A)39
B)59
C)35
D)19
E)15
A)39
B)59
C)35
D)19
E)15

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
38
The element in this list with chemical properties similar to magnesium is
A)sodium.
B)chlorine.
C)boron.
D)strontium.
E)carbon.
A)sodium.
B)chlorine.
C)boron.
D)strontium.
E)carbon.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
39
How many protons are in an isotope of sodium with a mass number of 25?
A)25
B)15
C)11
D)14
E)32
A)25
B)15
C)11
D)14
E)32

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
40
According to the Atomic Theory,
A)atoms are neither created nor destroyed during a chemical reaction.
B)atoms of the same element combine to form compounds.
C)all atoms are different.
D)all matter is made up of tiny particles called electrons.
E)a compound can contain different numbers of atoms as long as it has the same kinds of atoms.
A)atoms are neither created nor destroyed during a chemical reaction.
B)atoms of the same element combine to form compounds.
C)all atoms are different.
D)all matter is made up of tiny particles called electrons.
E)a compound can contain different numbers of atoms as long as it has the same kinds of atoms.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following electron configurations is impossible?
A)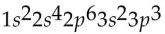
B)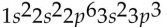
C)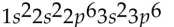
D)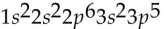
E)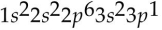
A)
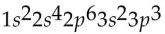
B)
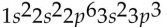
C)
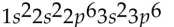
D)
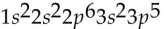
E)
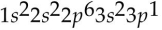

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
42
Given the following: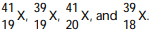 Which are isotopes of each other?
Which are isotopes of each other?
A)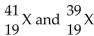 are isotopes of each other.
are isotopes of each other.
B)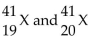 are isotopes of each other; and
are isotopes of each other; and 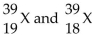 are isotopes of each other.
are isotopes of each other.
C)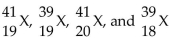 are isotopes of each other.
are isotopes of each other.
D)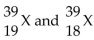 are isotopes of each other.
are isotopes of each other.
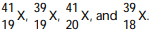 Which are isotopes of each other?
Which are isotopes of each other?A)
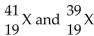 are isotopes of each other.
are isotopes of each other.B)
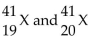 are isotopes of each other; and
are isotopes of each other; and 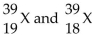 are isotopes of each other.
are isotopes of each other.C)
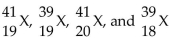 are isotopes of each other.
are isotopes of each other.D)
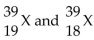 are isotopes of each other.
are isotopes of each other.
Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
43
Chlorine has two naturally occurring isotopes. The isotope Cl-35 (mass = 35.0 amu)makes up 75.8% of the sample, and the isotope Cl-37 (mass = 37.0 amu)makes up 24.2% of the sample. What is the average atomic
Mass for chlorine?
A)36.6 amu
B)35 amu
C)36.0 amu
D)35.521 amu
E)35.5 amu
Mass for chlorine?
A)36.6 amu
B)35 amu
C)36.0 amu
D)35.521 amu
E)35.5 amu

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
44
The number of electrons in the outer energy level of a neutral atom of boron (atomic number 5)is
A)2.
B)3.
C)5.
D)8.
E)10.
A)2.
B)3.
C)5.
D)8.
E)10.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
45
What is the electron configuration for aluminum?
A)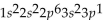
B)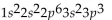
C)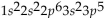
D)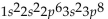
E)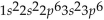
A)
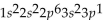
B)
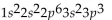
C)
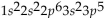
D)
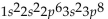
E)
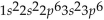

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
46
The number of orbitals in the 4d sublevel is
A)1.
B)2.
C)3.
D)4.
E)5.
A)1.
B)2.
C)3.
D)4.
E)5.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
47
What is the abbreviated electron configuration for nickel (atomic number 28)?
A)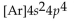
B)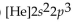
C)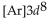
D)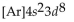
E)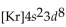
A)
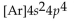
B)
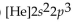
C)
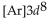
D)
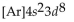
E)
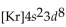

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
48
What element has the electron configuration 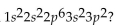
A)carbon
B)silicon
C)sulfur
D)oxygen
E)iron
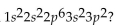
A)carbon
B)silicon
C)sulfur
D)oxygen
E)iron

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
49
What is the element with the electron configuration 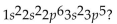
A)Cl
B)S
C)F
D)Ar
E)Be
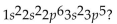
A)Cl
B)S
C)F
D)Ar
E)Be

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
50
The number of electron levels in a magnesium atom is
A)1.
B)2.
C)3.
D)4.
E)5.
A)1.
B)2.
C)3.
D)4.
E)5.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
51
The correct symbol for the isotope of potassium with 22 neutrons is
A)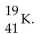
B)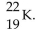
C)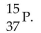
D)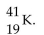
E)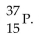
A)
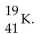
B)
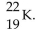
C)
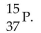
D)
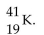
E)
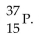

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
52
The atomic mass of an element is equal to
A)its mass number.
B)the average mass of all of the naturally occurring isotopes of the element.
C)its atomic number.
D)one-twelfth of the mass of a carbon-12 atom.
E)a weighted average mass of all of the naturally occurring isotopes of the element.
A)its mass number.
B)the average mass of all of the naturally occurring isotopes of the element.
C)its atomic number.
D)one-twelfth of the mass of a carbon-12 atom.
E)a weighted average mass of all of the naturally occurring isotopes of the element.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
53
The electron configuration of an atom shows
A)a description of the shape of each energy level.
B)the number of isotopes possible.
C)a diagram of an atomic nucleus.
D)the number of electrons in each energy level and sublevel.
E)the maximum number of electrons each energy level can hold.
A)a description of the shape of each energy level.
B)the number of isotopes possible.
C)a diagram of an atomic nucleus.
D)the number of electrons in each energy level and sublevel.
E)the maximum number of electrons each energy level can hold.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
54
What is the electron configuration for potassium (atomic number 19)?
A)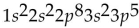
B)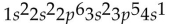
C)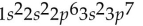
D)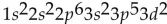
E)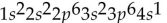
A)
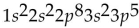
B)
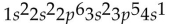
C)
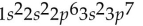
D)
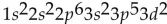
E)
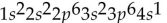

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
55
Isotopes are atoms of the same element that have
A)the same atomic numbers but different numbers of electrons.
B)the same atomic number but different numbers of neutrons.
C)the same atomic mass but different numbers of protons.
D)the same atomic numbers but different numbers of protons.
E)different atomic numbers.
A)the same atomic numbers but different numbers of electrons.
B)the same atomic number but different numbers of neutrons.
C)the same atomic mass but different numbers of protons.
D)the same atomic numbers but different numbers of protons.
E)different atomic numbers.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
56
What is the element with the abbreviated electron configuration 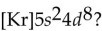
A)Pd
B)Kr
C)Pt
D)Xe
E)Ni
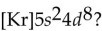
A)Pd
B)Kr
C)Pt
D)Xe
E)Ni

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
57
The maximum number of electrons that may occupy the third energy level is
A)18.
B)32.
C)8.
D)10.
E)2.
A)18.
B)32.
C)8.
D)10.
E)2.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
58
What is the correct electron configuration for the lithium atom?
A)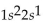
B)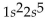
C)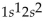
D)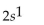
E)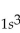
A)
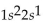
B)
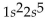
C)
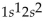
D)
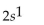
E)
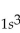

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
59
Silicon has three naturally occurring isotopes: Si-28 (mass 28.0 amu); Si-29 (mass = 29.0 amu)and Si-30 (mass = 30.0 amu). If the average atomic mass of silicon is 28.1 amu, which isotope is the most abundant?
A)Si-29
B)Si-28
C)Si-30
D)All isotopes have the same natural abundance.
A)Si-29
B)Si-28
C)Si-30
D)All isotopes have the same natural abundance.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is NOT true for the atoms 13N, 14N, and 15N?
A) They all have the same mass number.
B) They all have 7 protons.
C) They all have 7 electrons.
D) They are isotopes.
E) They all have the same atomic number.
A) They all have the same mass number.
B) They all have 7 protons.
C) They all have 7 electrons.
D) They are isotopes.
E) They all have the same atomic number.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
61
Of the elements: B, C, F, Li, and Na, the element with the smallest atomic radius is
A)B.
B)C.
C)F.
D)Li.
E)Na.
A)B.
B)C.
C)F.
D)Li.
E)Na.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
62
The number of valence electrons found in an atom of a Group A element is equal to
A)eight.
B)its mass number.
C)its atomic number.
D)eight minus the group number.
E)its group number.
A)eight.
B)its mass number.
C)its atomic number.
D)eight minus the group number.
E)its group number.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
63
Valence electrons are electrons located
A)throughout the atom.
B)in the first three energy levels of an atom.
C)in the innermost energy level of an atom.
D)in the nucleus of an atom.
E)in the outermost energy level of an atom.
A)throughout the atom.
B)in the first three energy levels of an atom.
C)in the innermost energy level of an atom.
D)in the nucleus of an atom.
E)in the outermost energy level of an atom.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
64
Of the elements: Li, Na, K, Rb, and Cs, the element with the most metallic character is
A)Li.
B)Na.
C)K.
D)Rb.
E)Cs.
A)Li.
B)Na.
C)K.
D)Rb.
E)Cs.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
65
Write in the electronic configuration for the atom shown.
Chlorine
Chlorine

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
66
The number of dots in the electron dot structure of nitrogen is
A)one.
B)two.
C)three.
D)four.
E)five.
A)one.
B)two.
C)three.
D)four.
E)five.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
67
The ionization energy of atoms
A)decreases going down within a group.
B)increases going down within a group.
C)does not change going down within a group.
D)decreases going across a period.
A)decreases going down within a group.
B)increases going down within a group.
C)does not change going down within a group.
D)decreases going across a period.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
68
Write in the electronic configuration for the atom shown.
Sodium
Sodium

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
69
The number of dots in the electron dot structure of carbon is
A)one.
B)two.
C)three.
D)four.
E)five.
A)one.
B)two.
C)three.
D)four.
E)five.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
70
Of the elements: B, C, F, Li, and Na, the element with the most metallic character is
A)B.
B)C.
C)F.
D)Li.
E)Na.
A)B.
B)C.
C)F.
D)Li.
E)Na.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
71
In an electron-dot structure of an element, the dots are used to represent
A)the electron arrangement.
B)the electrons that the element will gain when it forms a compound.
C)only the electrons that will participate in bond formation.
D)all of the electrons in the atom.
E)the valence electrons.
A)the electron arrangement.
B)the electrons that the element will gain when it forms a compound.
C)only the electrons that will participate in bond formation.
D)all of the electrons in the atom.
E)the valence electrons.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
72
How many valence electrons are in the electron-dot structures for the elements in group 3A(13)?
A)1
B)2
C)3
D)4
E)6
A)1
B)2
C)3
D)4
E)6

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
73
Of the elements: B, C, F, Li, and Na, the element with the least metallic character is
A)B.
B)C.
C)F.
D)Li.
E)Na.
A)B.
B)C.
C)F.
D)Li.
E)Na.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
74
The atomic size of atoms
A)decreases going across a period.
B)decreases going down within a group.
C)does not change going across a period.
D)increases going across a period.
A)decreases going across a period.
B)decreases going down within a group.
C)does not change going across a period.
D)increases going across a period.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
75
Ionization energy is
A)the energy an ion acquires from an electron.
B)higher for potassium than for lithium.
C)highest for metals in Group 1A (1).
D)the energy needed to remove an electron from the outermost energy level.
E)higher for chlorine than for fluorine.
A)the energy an ion acquires from an electron.
B)higher for potassium than for lithium.
C)highest for metals in Group 1A (1).
D)the energy needed to remove an electron from the outermost energy level.
E)higher for chlorine than for fluorine.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
76
Write in the electronic configuration for the atom shown.
Argon
Argon

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following is the correct electron-dot structure for carbon?
A)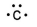
B)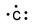
C)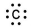
D)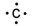
E)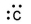
A)
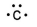
B)
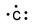
C)
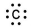
D)
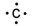
E)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
78
Of the elements: B, C, F, Li, and Na, the element with the highest ionization energy is
A)B.
B)C.
C)F.
D)Li.
E)Na.
A)B.
B)C.
C)F.
D)Li.
E)Na.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
79
Of the elements: B, C, F, Li, and Na, the element with the smallest ionization energy is
A)B.
B)C.
C)F.
D)Li.
E)Na.
A)B.
B)C.
C)F.
D)Li.
E)Na.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
80
Of the elements: B, C, F, Li, and Na, the element with the largest atomic radius is
A)B.
B)C.
C)F.
D)Li.
E)Na.
A)B.
B)C.
C)F.
D)Li.
E)Na.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck


