Deck 13: Acids and Bases: the Molecules Responsible for Sour and Bitter
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/69
Play
Full screen (f)
Deck 13: Acids and Bases: the Molecules Responsible for Sour and Bitter
1
Which of the following substances is a base?
A)Sodium hydroxide
B)Potassium hydroxide
C)Sodium bicarbonate
D)Ammonia
E)All of the above
A)Sodium hydroxide
B)Potassium hydroxide
C)Sodium bicarbonate
D)Ammonia
E)All of the above
All of the above
2
Water can act as _____.
A)a proton donor
B)a proton acceptor
C)either a proton donor or a proton acceptor
D)neither a proton donor nor a proton acceptor
A)a proton donor
B)a proton acceptor
C)either a proton donor or a proton acceptor
D)neither a proton donor nor a proton acceptor
either a proton donor or a proton acceptor
3
Which of these is the best definition of a Bronsted-Lowry base?
A)Any substance that produces H+ ions in solution.
B)Any substance that produces OH − ions in solution.
C)Any substance that accepts a lone pair of electrons.
D)Any substance that neutralizes an acid in solution.
E)Any substance that accepts H+ ion in solution.
A)Any substance that produces H+ ions in solution.
B)Any substance that produces OH − ions in solution.
C)Any substance that accepts a lone pair of electrons.
D)Any substance that neutralizes an acid in solution.
E)Any substance that accepts H+ ion in solution.
Any substance that accepts H+ ion in solution.
4
Which of these compounds can not act as a Bronsted-Lowry base?
A)NH4+
B)OH −
C)CH3COO −
D)H2O
E)NH3
A)NH4+
B)OH −
C)CH3COO −
D)H2O
E)NH3

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
5
The formula of hydronium ion is _____.
A)OH −
B)OH+
C)H3O+
D)H3O −
E)none of the above
A)OH −
B)OH+
C)H3O+
D)H3O −
E)none of the above

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
6
Which of these are properties of acids?
I. Sour
II. Turn litmus blue
III. Dissolve some metals
IV. Slippery
A)I only
B)III only
C)I and II
D)I and III
E)II, III and IV
I. Sour
II. Turn litmus blue
III. Dissolve some metals
IV. Slippery
A)I only
B)III only
C)I and II
D)I and III
E)II, III and IV

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
7
Which of these is the Bronsted-Lowry acid in this chemical equation?
H2O + CN − → HCN + OH −
A)H3O+
B)H2O
C)H2SO4
D)CN −
E)OH −
H2O + CN − → HCN + OH −
A)H3O+
B)H2O
C)H2SO4
D)CN −
E)OH −

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
8
Which of these substances is a strong acid?
A)HCN
B)H2PO3
C)HCl
D)HNO2
E)H2CO3
A)HCN
B)H2PO3
C)HCl
D)HNO2
E)H2CO3

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
9
Which of these statements about strong acids is correct ?
A)Acids which are concentrated.
B)Acids which have a very high pH.
C)Acids which dissociate 100% into ions.
D)Acids which dissociate very little into ions.
E)Both B and C are correct.
A)Acids which are concentrated.
B)Acids which have a very high pH.
C)Acids which dissociate 100% into ions.
D)Acids which dissociate very little into ions.
E)Both B and C are correct.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
10
The formula of the hydroxide ion is:
A)OH −
B)OH+
C)H3O+
D)H3O −
E)none of the above
A)OH −
B)OH+
C)H3O+
D)H3O −
E)none of the above

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
11
Which of these substances is a base?
A)NaHCO3
B)HCl
C)HNO3
D)H3PO4
E)CH3COOH
A)NaHCO3
B)HCl
C)HNO3
D)H3PO4
E)CH3COOH

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
12
Which of these acids is a component in vinegar?
A)H2SO4
B)HCl
C)HNO3
D)NaOH
E)CH3COOH
A)H2SO4
B)HCl
C)HNO3
D)NaOH
E)CH3COOH

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
13
Which of these is the Bronsted-Lowry base in this chemical equation?
H2O + CH3COOH CH3COO − + H3O+
CH3COO − + H3O+
A)H3O+
B)H2O
C)CH3COOH
D) CH3COO-
E) OH-
H2O + CH3COOH
 CH3COO − + H3O+
CH3COO − + H3O+A)H3O+
B)H2O
C)CH3COOH
D) CH3COO-
E) OH-

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
14
Which of these substances is a weak base?
A)HCN
B)CH3COOH
C)HCl
D)HCOOH
E)C5H5N
A)HCN
B)CH3COOH
C)HCl
D)HCOOH
E)C5H5N

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
15
Which of these are properties of bases?
I. Sour
II. Turn litmus blue
III. Dissolve some metals
IV. Slippery
A)I only
B)III only
C)I and II
D)I and III
E)II and IV
I. Sour
II. Turn litmus blue
III. Dissolve some metals
IV. Slippery
A)I only
B)III only
C)I and II
D)I and III
E)II and IV

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
16
Which of these compounds can not act as a Bronsted-Lowry acid?
A)H3O+
B)H2O
C)CH3COOH
D)MgO
E)NH3
A)H3O+
B)H2O
C)CH3COOH
D)MgO
E)NH3

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
17
Which of these is the Bronsted-Lowry base in this chemical equation?
HSO4 − + OH − SO42 − + H2O
SO42 − + H2O
A)HSO4 −
B)H2O
C)S
D)OH −
E)NH3
HSO4 − + OH −
 SO42 − + H2O
SO42 − + H2OA)HSO4 −
B)H2O
C)S
D)OH −
E)NH3

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
18
Which of these is the best definition of a Bronsted-Lowry acid?
A)Any substance that donates H+ ions in solution.
B)Any substance that produces OH − ions in solution.
C)Any substance that accepts a lone pair of electrons.
D)Any substance that neutralizes a base in solution.
E)Any substance that accepts H+ ion in solution.
A)Any substance that donates H+ ions in solution.
B)Any substance that produces OH − ions in solution.
C)Any substance that accepts a lone pair of electrons.
D)Any substance that neutralizes a base in solution.
E)Any substance that accepts H+ ion in solution.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
19
Which of these is the best definition of an Arrehenius base?
A)Any substance that produces H+ ions in solution.
B)Any substance that produces OH − ions in solution.
C)Any substance that accepts a lone pair of electrons.
D)Any substance that neutralizes an acid in solution.
E)Any substance that accepts H+ ion in solution.
A)Any substance that produces H+ ions in solution.
B)Any substance that produces OH − ions in solution.
C)Any substance that accepts a lone pair of electrons.
D)Any substance that neutralizes an acid in solution.
E)Any substance that accepts H+ ion in solution.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
20
Which of these substances is an acid?
A)NaHCO3
B)NaOH
C)NH3
D)KOH
E)CH3COOH
A)NaHCO3
B)NaOH
C)NH3
D)KOH
E)CH3COOH

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
21
Which of these acids is responsible for the tartness of vinegar?
A)Muriatic
B)Citric
C)Sulfuric
D)Acetic
E)Benzoic
A)Muriatic
B)Citric
C)Sulfuric
D)Acetic
E)Benzoic

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
22
Which of these has the greatest [H3O+]?
A)pH = 2
B)pH = 5
C)pH = 7
D)pH = 11
E)pH = 13
A)pH = 2
B)pH = 5
C)pH = 7
D)pH = 11
E)pH = 13

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
23
Which of these has the smallest [H3O+]?
A)pH = 2
B)pH = 5
C)pH = 7
D)pH = 11
E)pH = 13
A)pH = 2
B)pH = 5
C)pH = 7
D)pH = 11
E)pH = 13

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
24
Which of these substances is represented in the diagram?
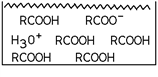
A)A weak acid
B)A weak base
C)A strong acid
D)A strong base
E)A salt

A)A weak acid
B)A weak base
C)A strong acid
D)A strong base
E)A salt

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
25
What is the pH of pure water at 25 oC?
A)0
B)1
C)10
D)14
E)none of the above
A)0
B)1
C)10
D)14
E)none of the above

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
26
Which of these statements concerning pure water is incorrect ?
A)The pH is 7 at 25 oC.
B)It contains no ions.
C)It is neutral.
D)It can act as a proton acceptor.
A)The pH is 7 at 25 oC.
B)It contains no ions.
C)It is neutral.
D)It can act as a proton acceptor.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
27
Which of these acids forms when wine is left exposed to oxygen for extended periods?
A)Muriatic
B)Citric
C)Sulfuric
D)Acetic
E)Benzoic
A)Muriatic
B)Citric
C)Sulfuric
D)Acetic
E)Benzoic

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
28
Which of these substances is a weak acid?
A)NH3
B)HCOOH
C)HCl
D)HNO3
E)C5H5N
A)NH3
B)HCOOH
C)HCl
D)HNO3
E)C5H5N

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
29
Which of these statements concerning pH is incorrect ?
A)pH is based on the [H3O+] in solution.
B)If [H3O+] = 1.0 × 10 − 13, then the solution is basic.
C)pH = 3.0 is ten times more acidic than pH = 2.
D)If the pH > 7 then the solution is basic.
E)Pure water has [H3O+] = 1.0 × 10 − 7.
A)pH is based on the [H3O+] in solution.
B)If [H3O+] = 1.0 × 10 − 13, then the solution is basic.
C)pH = 3.0 is ten times more acidic than pH = 2.
D)If the pH > 7 then the solution is basic.
E)Pure water has [H3O+] = 1.0 × 10 − 7.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
30
What is the pH of a 1.0 M solution of a strong acid?
A)0
B)1.00
C)13.00
D)14.00
E)Can't tell from the given information.
A)0
B)1.00
C)13.00
D)14.00
E)Can't tell from the given information.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
31
What is the pH of a 1.0 M solution of a weak acid?
A)0
B)1.00
C)13.00
D)14.00
E)Can't tell from the given information.
A)0
B)1.00
C)13.00
D)14.00
E)Can't tell from the given information.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
32
Which of these acids is responsible for the sourness in many fruits?
A)Muriatic
B)Citric
C)Sulfuric
D)Acetic
E)Benzoic
A)Muriatic
B)Citric
C)Sulfuric
D)Acetic
E)Benzoic

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
33
What is the pH of a 1.0 M solution of a strong base?
A)0
B)1.00
C)13.00
D)14.00
E)Can't tell from the given information.
A)0
B)1.00
C)13.00
D)14.00
E)Can't tell from the given information.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
34
Which of these statements concerning pH is incorrect ?
A)pH is based on the [H3O+] in solution.
B)If [H3O+] = 1.0 × 10 − 4, the solution is acidic.
C)If pH = 7.0 then the solution is neutral.
D)If the pH
E)Pure water has [H3O+] = 1.0 × 10-7 M.
A)pH is based on the [H3O+] in solution.
B)If [H3O+] = 1.0 × 10 − 4, the solution is acidic.
C)If pH = 7.0 then the solution is neutral.
D)If the pH
E)Pure water has [H3O+] = 1.0 × 10-7 M.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
35
Which of these statements about weak bases is correct ?
A)Bases which are concentrated.
B)Bases which have a very high pH.
C)Bases which dissociate 100% into ions.
D)Bases which dissociate very little into ions.
E)Both B and C are correct.
A)Bases which are concentrated.
B)Bases which have a very high pH.
C)Bases which dissociate 100% into ions.
D)Bases which dissociate very little into ions.
E)Both B and C are correct.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
36
Which of these substances is represented in the diagram?
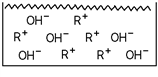
A)A weak acid
B)A weak base
C)A strong acid
D)A strong base
E)A salt

A)A weak acid
B)A weak base
C)A strong acid
D)A strong base
E)A salt

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
37
Which of these sets indicating relative acid strength is correct ?
A)H2CO3> NH3
B)HCOOH > H2SO4
C)C5H5N > H3PO4
D)KOH > CH3COOH
E)C6H5COOH > HNO3
A)H2CO3> NH3
B)HCOOH > H2SO4
C)C5H5N > H3PO4
D)KOH > CH3COOH
E)C6H5COOH > HNO3

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
38
Compared to a solution that has a pH of 2.00, a solution that has a pH of 4.00:
A)contains twice as much hydronium ion.
B)contains half as much hydronium ion.
C)contains 100 times as much hydronium ion.
D)contains .01 times as much hydronium ion.
E)none of the above
A)contains twice as much hydronium ion.
B)contains half as much hydronium ion.
C)contains 100 times as much hydronium ion.
D)contains .01 times as much hydronium ion.
E)none of the above

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
39
Compared to a solution that has a pH of 2.00, a solution that has a pH of 4.00:
A)contains twice as much hydroxide ion.
B)contains half as much hydroxide ion.
C)contains 100 times as much hydroxide ion.
D)contains .01 times as much hydroxide ion.
E)none of the above
A)contains twice as much hydroxide ion.
B)contains half as much hydroxide ion.
C)contains 100 times as much hydroxide ion.
D)contains .01 times as much hydroxide ion.
E)none of the above

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
40
Which of these sets indicating relative acid strength is correct ?
A)NH3 > H2CO3
B)HCOOH > H2SO4
C)HCl > CH3COOH
D)C6H6COOH > HNO3
E)C5H5N > H3PO4
A)NH3 > H2CO3
B)HCOOH > H2SO4
C)HCl > CH3COOH
D)C6H6COOH > HNO3
E)C5H5N > H3PO4

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
41
Which of these acids is responsible for the apple flavor of some wines?
A)Malic
B)Citric
C)Tartaric
D)Acetic
E)Lactic
A)Malic
B)Citric
C)Tartaric
D)Acetic
E)Lactic

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
42
Which of these pairs represent the products of this dissociation in water?
NaHCO3 = ____ + ____
A)NaOH, CO2
B)OH − , NaCO+
C)NaH+2, CO32 +
D)Na+, HCO3 −
E)H2O, CO
NaHCO3 = ____ + ____
A)NaOH, CO2
B)OH − , NaCO+
C)NaH+2, CO32 +
D)Na+, HCO3 −
E)H2O, CO

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
43
Which of these acids is found in stomach acid?
A)Malic
B)Hydrochloric
C)Phosphoric
D)Acetic
E)Lactic
A)Malic
B)Hydrochloric
C)Phosphoric
D)Acetic
E)Lactic

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
44
Which of these substances is most basic?
A)Lemons
B)Corn
C)Soft drinks
D)Blood
E)Wine
A)Lemons
B)Corn
C)Soft drinks
D)Blood
E)Wine

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
45
Which of these acids is not found in wines?
A)Lactic
B)Citric
C)Tartaric
D)Acetic
E)Salicylic
A)Lactic
B)Citric
C)Tartaric
D)Acetic
E)Salicylic

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
46
Which of these substances is most acidic?
A)Lemons
B)Pure water
C)Milk of magnesia
D)Blood
E)Wine
A)Lemons
B)Pure water
C)Milk of magnesia
D)Blood
E)Wine

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
47
Which of these two acids are found in most soft drinks?
A)Acetic and citric
B)Lactic and citric
C)Malic and lactic
D)Carbonic and phosphoric
E)Hydrochloric and phosphoric
A)Acetic and citric
B)Lactic and citric
C)Malic and lactic
D)Carbonic and phosphoric
E)Hydrochloric and phosphoric

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
48
Which of these equations best represents the reaction that takes place in the stomach when the antacid calcium carbonate is taken?
A)NaHCO3 + H2O + CO
B)CaCO3 = H2O + CO
C)CO32 − + 2H3O+ = 3H2O + CO2
D)Ca2+ + 2H3O+ = 3H2O + CaO
E)CaCO3 + H3O+ = Ca(OH)2 + CO2
A)NaHCO3 + H2O + CO
B)CaCO3 = H2O + CO
C)CO32 − + 2H3O+ = 3H2O + CO2
D)Ca2+ + 2H3O+ = 3H2O + CaO
E)CaCO3 + H3O+ = Ca(OH)2 + CO2

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
49
Rank these substances in increasing acidity (least acidic to most acidic).
1.0 M HCOOH, 1.0 M NaHCO3, 1.0 M H2SO4
A)1.0 M HCOOH
B)1.0 M NaHCO3
C)1.0 M H2SO4
D)1.00 M H2SO4
E)1.00 M NaHCO3
1.0 M HCOOH, 1.0 M NaHCO3, 1.0 M H2SO4
A)1.0 M HCOOH
B)1.0 M NaHCO3
C)1.0 M H2SO4
D)1.00 M H2SO4
E)1.00 M NaHCO3

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
50
Which of these is the active ingredient in aspirin?
A)Salicylic acid
B)Acetylsalicylic acid
C)Acetic acid
D)Lactic acid
A)Salicylic acid
B)Acetylsalicylic acid
C)Acetic acid
D)Lactic acid

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
51
Which of these pairs represent the products of this dissociation in water?
Mg(OH)2 = ____ + ____
A)MgOH, H2
B)OH − , MgOH+
C)Mg+2, 2OH −
D)Mg+, 2OH −
E)OH2 − , Mg+
Mg(OH)2 = ____ + ____
A)MgOH, H2
B)OH − , MgOH+
C)Mg+2, 2OH −
D)Mg+, 2OH −
E)OH2 − , Mg+

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
52
Which of these acids is responsible for the buttery flavor of some wines?
A)Malic
B)Citric
C)Tartaric
D)Acetic
E)Lactic
A)Malic
B)Citric
C)Tartaric
D)Acetic
E)Lactic

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
53
Which of these substances would have the lowest pH?
A)1.0 M CH3COOH
B)1.0 M HNO3
C)1.0 M NH3
D)1.0 M HCOOH
E)1.0 M NaOH
A)1.0 M CH3COOH
B)1.0 M HNO3
C)1.0 M NH3
D)1.0 M HCOOH
E)1.0 M NaOH

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
54
Which of these is not a base?
A)HCOOH
B)NaHCO3
C)NaOH
D)CaCO3
E)Mg(OH)2
A)HCOOH
B)NaHCO3
C)NaOH
D)CaCO3
E)Mg(OH)2

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
55
Which of these substances would have the highest pH?
A)1.0 M CH3COOH
B)1.0 M HNO3
C)1.0 M NH3
D)1.0 M HCOOH
E)1.0 M NaOH
A)1.0 M CH3COOH
B)1.0 M HNO3
C)1.0 M NH3
D)1.0 M HCOOH
E)1.0 M NaOH

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
56
Which of these substances is most acidic?
A)Pure water
B)Corn
C)Soft drinks
D)Blood
E)Beer
A)Pure water
B)Corn
C)Soft drinks
D)Blood
E)Beer

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
57
Which of these is the primary ingredient in baking soda?
A)NaOH
B)Mg(OH)2
C)Al(OH)3
D)CaCO3
E)NaHCO3
A)NaOH
B)Mg(OH)2
C)Al(OH)3
D)CaCO3
E)NaHCO3

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
58
Rank these compounds in increasing acidity (least acidic to most acidic).
1.0 M HCOOH, 1.0 M HCl, 1.0 M NH3 , 1.0 M NaOH
A)1.0 M HCOOH
B)1.0 M NH3
C)1.0 M NaOH
D)1.00 M NaOH
E)1.00 M NH3
1.0 M HCOOH, 1.0 M HCl, 1.0 M NH3 , 1.0 M NaOH
A)1.0 M HCOOH
B)1.0 M NH3
C)1.0 M NaOH
D)1.00 M NaOH
E)1.00 M NH3

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
59
Which of these substances is most basic?
A)Lemons
B)Pure water
C)Milk of magnesia
D)Blood
E)Wine
A)Lemons
B)Pure water
C)Milk of magnesia
D)Blood
E)Wine

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
60
Which of these acids is a parent molecule for making aspirin?
A)Muriatic
B)Citric
C)Benzoic
D)Acetic
E)Salicylic
A)Muriatic
B)Citric
C)Benzoic
D)Acetic
E)Salicylic

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
61
Which of these methods of reducing SO2 emissions employs spraying limestone and water in coal smokestacks?
A)Crushing and washing
B)Vaporizing and condensing
C)Subliming
D)Flue gas scrubbers
E)None of these
A)Crushing and washing
B)Vaporizing and condensing
C)Subliming
D)Flue gas scrubbers
E)None of these

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
62
Which of these statements about acid rain is correct?
A)Acid rain has destroyed all marine life in entire lakes.
B)Acid rain slows the rate of tree growth.
C)Acid rain accelerates the rusting process.
D)Acid rain can effectively remove nutrients from soil.
E)All of the above are correct.
A)Acid rain has destroyed all marine life in entire lakes.
B)Acid rain slows the rate of tree growth.
C)Acid rain accelerates the rusting process.
D)Acid rain can effectively remove nutrients from soil.
E)All of the above are correct.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
63
Which of these represents the average pH of ordinary rain water?
A)1
B)2.8
C)3.5
D)5.6
E)7.0
A)1
B)2.8
C)3.5
D)5.6
E)7.0

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
64
Which of these gases combine with water to form acid rain?
I. SO2
II. NO2
III. H2
IV. N2
V. Cl2
A)I only
B)II only
C)I and II
D)I and IV
E)I, III and V
I. SO2
II. NO2
III. H2
IV. N2
V. Cl2
A)I only
B)II only
C)I and II
D)I and IV
E)I, III and V

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
65
Which of these gases is targeted by the 1990 Clean Air Act for a 50% reduction of emission by 2010?
A)SO2
B)CO2
C)NO2
D)Cl2
E)N2
A)SO2
B)CO2
C)NO2
D)Cl2
E)N2

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
66
The most acidic rainfall in the United States occurs in _____.
A)the northwest region.
B)the southwest region
C)the southeast region
D)the northeast region
E)the midwest.
A)the northwest region.
B)the southwest region
C)the southeast region
D)the northeast region
E)the midwest.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
67
Rain is naturally acidic as a result of ____ gas reacting with the water.
A)SO2
B)CO2
C)NO2
D)Cl2
E)N2
A)SO2
B)CO2
C)NO2
D)Cl2
E)N2

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
68
The combustion of which of these fuels is the most significant contributor in the formation of acid rain?
A)Oil
B)Coal
C)Natural gas
D)Geothermal
E)Nuclear
A)Oil
B)Coal
C)Natural gas
D)Geothermal
E)Nuclear

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
69
Which of these is not a method used by utilities to reduce sulfur dioxide emissions?
A)Burning low-sulfur coal.
B)Crushing and washing coal to remove sulfur.
C)Burning coal at high temperatures to break down the sulfur.
D)Employing flue gas scrubbers in smoke stacks which traps sulfur dioxide.
E)All of these are current methods utilities are using to lower SO2 emissions.
A)Burning low-sulfur coal.
B)Crushing and washing coal to remove sulfur.
C)Burning coal at high temperatures to break down the sulfur.
D)Employing flue gas scrubbers in smoke stacks which traps sulfur dioxide.
E)All of these are current methods utilities are using to lower SO2 emissions.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck


