Deck 5: Quantum Mechanics and Atomic Structure
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/33
Play
Full screen (f)
Deck 5: Quantum Mechanics and Atomic Structure
1
Which of the following sets of quantum numbers is allowed for an electron in a one electron atom?
A) n=4, l=3, m=3, ms=0
B) n=3, l=1, m=2, ms= -1/2
C) n=2, l=0, m=1, ms=1/2
D) n=2, l=3, m=3, ms=1/2
E) n=6, l=5, m= -3, ms= -1/2
A) n=4, l=3, m=3, ms=0
B) n=3, l=1, m=2, ms= -1/2
C) n=2, l=0, m=1, ms=1/2
D) n=2, l=3, m=3, ms=1/2
E) n=6, l=5, m= -3, ms= -1/2
E
2
Which of the follow are possible quantum numbers for a 3d wave function in a hydrogen atom?
A) n=2 l=3 m=3
B) n=3 l=0 m=0
C) n=3 l=3 m= -2
D) n=3 l=2 m= -1
E) none of the above
A) n=2 l=3 m=3
B) n=3 l=0 m=0
C) n=3 l=3 m= -2
D) n=3 l=2 m= -1
E) none of the above
D
3
How many radial and angular nodes does a 4p orbital for a one electron atom have?
A) 3 radial, 1 angular
B) 2 radial, 2 angular
C) 2 radial, 1 angular
D) 2 radial, 0 angular
E) 1 radial, 1 angular
A) 3 radial, 1 angular
B) 2 radial, 2 angular
C) 2 radial, 1 angular
D) 2 radial, 0 angular
E) 1 radial, 1 angular
C
4
How many possible sets of quantum numbers (n, l, m, ms) are there with n=4?
A) 1
B) 4
C) 8
D) 16
E) 32
A) 1
B) 4
C) 8
D) 16
E) 32

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
5
The most probable distance for an electron in a 1s wavefuction in hydrogen is at a distance equal to ao/Z. Given this information you would predict that Li2+ would be
A) 2 times larger than hydrogen
B) 2 times smaller than hydrogen
C) 3 times larger than hydrogen
D) 3 times smaller than hydrogen
E) no way to guess exactly due to shielding effects
A) 2 times larger than hydrogen
B) 2 times smaller than hydrogen
C) 3 times larger than hydrogen
D) 3 times smaller than hydrogen
E) no way to guess exactly due to shielding effects

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
6
The ground state 1s electron in which atom has an average distance closest to the nucleus?
A) H
B) He+
C) He
D) a & b are the same and closest
E) c & d are the same and closest
A) H
B) He+
C) He
D) a & b are the same and closest
E) c & d are the same and closest

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
7
For C would the Zeff be larger for the 2s or the 2p Hartree orbital?
A) Zeff(2s) would be larger
B) Zeff(2p) would be larger
C) Zeff(2s) would be equal to Zeff(2p)
D) it depends on the spin
A) Zeff(2s) would be larger
B) Zeff(2p) would be larger
C) Zeff(2s) would be equal to Zeff(2p)
D) it depends on the spin

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
8
Which is the best estimate for Zeff(1s) for the Hartree orbitals for He?
A) 1.0
B) 1.7
C) 2.0
D) 2.3
E) 3
A) 1.0
B) 1.7
C) 2.0
D) 2.3
E) 3

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
9
Given that Zeff(2s) in Li is 1.26, estimate the energy of the 2s electron in Li in Rhydberg units.
A) -0.40
B) -0.63
C) -0.79
D) -1.26
E) -4.0
A) -0.40
B) -0.63
C) -0.79
D) -1.26
E) -4.0

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
10
What is the electronic configuration for Si?
A) [He]2s22p2
B) [Ne]2p4
C) [Ne]3p4
D) [Ne]3s23p2
E) [Na]3s22p2
A) [He]2s22p2
B) [Ne]2p4
C) [Ne]3p4
D) [Ne]3s23p2
E) [Na]3s22p2

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
11
Which group of atoms and ions is isoelectronic?
A) O2-, F-, Ne, Li+
B) O2-, F-, Ne, Mg2+
C) Na+, Li+, Mg2+, Ca2+
D) F-, Cl-, Br-, Ar
E) F-, S2-, He
A) O2-, F-, Ne, Li+
B) O2-, F-, Ne, Mg2+
C) Na+, Li+, Mg2+, Ca2+
D) F-, Cl-, Br-, Ar
E) F-, S2-, He

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
12
Given that the electronic configuration of Cu is [Ar]3d104s1 what is the electronic configuration of Cu+?
A) [Ar]3d10
B) [Ar]3d94s1
C) [Ar]3d104s2
D) [Ne]3s23p53d104s1
E) [Ne]3s13p63d104s1
A) [Ar]3d10
B) [Ar]3d94s1
C) [Ar]3d104s2
D) [Ne]3s23p53d104s1
E) [Ne]3s13p63d104s1

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
13
What is the electronic configuration of Sn?
A) [Kr]4s24d104p2
B) [Kr]5s25d105p2
C) [Kr]5s24d105p2
D) [Kr]4s25d104p2
E) [Kr]5s24d12
A) [Kr]4s24d104p2
B) [Kr]5s25d105p2
C) [Kr]5s24d105p2
D) [Kr]4s25d104p2
E) [Kr]5s24d12

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following would you expect to have unpaired electrons?
A) Be
B) Zn
C) Ne
D) Si
E) none of the above
A) Be
B) Zn
C) Ne
D) Si
E) none of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
15
What +2 ion has the electronic configuration [Xe]6s24f145d10?
A) Ba2+
B) Po2+
C) Pt2+
D) Pb2+
E) Hg2+
A) Ba2+
B) Po2+
C) Pt2+
D) Pb2+
E) Hg2+

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
16
A photoelectron spectrum of sodium excited with x-rays with an energy of 1254 eV shows two peaks at kinetics energies of 1190 eV and 1223 eV. These peaks are assigned to the sodium 2s and 2p electrons. What is the energy of the 2s electron in sodium?
A) -31 eV
B) -33 eV
C) -64 eV
D) -128 eV
E) -1190 eV
A) -31 eV
B) -33 eV
C) -64 eV
D) -128 eV
E) -1190 eV

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
17
The photoelectron spectra of Pd shows the 3d electrons to have an energy of -335 eV. Estimate the Zeff for this orbital in Pd.
A) 14.9
B) 22.2
C) 44.7
D) 46
E) 54.9
A) 14.9
B) 22.2
C) 44.7
D) 46
E) 54.9

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
18
The 2p level in fluorine has a binding energy of -12 eV. If a photoelectron spectrum of fluorine is taken with ultraviolet light with an energy of 21.2 eV, at what kinetic energy will the 2p electrons be observed?
A) 3.0 eV
B) 9.2 eV
C) 16.6 eV
D) 27.2 eV
E) 33.2 eV
A) 3.0 eV
B) 9.2 eV
C) 16.6 eV
D) 27.2 eV
E) 33.2 eV

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
19
Which will have the largest ionization potential?
A) Li
B) Li+
C) He
D) K
E) Cs
A) Li
B) Li+
C) He
D) K
E) Cs

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
20
In which of the following pairs does the second atom or ion has the larger radius?
A) He, Li+
B) F-, F
C) Br, Cl
D) Na, Mg
E) Na, Na+
A) He, Li+
B) F-, F
C) Br, Cl
D) Na, Mg
E) Na, Na+

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
21
Rank from smallest to largest in terms of atom/ionic radii.
A) S2-, Cl-, Ar, K+, Ca2+
B) Ca2+, K+, Ar, Cl-, S2-
C) Ca2+, S2-, K+, Cl-, Ar
D) Ar, K+, Cl-, Ca2+, S2-
E) Ar, Cl-, K+, S2-, Ca2+
A) S2-, Cl-, Ar, K+, Ca2+
B) Ca2+, K+, Ar, Cl-, S2-
C) Ca2+, S2-, K+, Cl-, Ar
D) Ar, K+, Cl-, Ca2+, S2-
E) Ar, Cl-, K+, S2-, Ca2+

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following would you expect to have the largest third ionization energy?
A) Mg
B) Si
C) Al
D) Ne
E) Ti
A) Mg
B) Si
C) Al
D) Ne
E) Ti

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
23
Which will be larger the first ionization energy of Ar or the second ionization energy of K?
A) E1 of Ar
B) E2 of K
C) they will be exactly equal
D) it depends on the shielding
A) E1 of Ar
B) E2 of K
C) they will be exactly equal
D) it depends on the shielding

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
24
Given that the first ionization energy O is lower than that of N what would you predict for the Zeff(2p) in these atoms?
A) Zeff(2p) is larger in N than in O
B) Zeff(2p) is larger in O than in N
C) they are exactly the same
D) the ionization energy cannot be related to Zeff(2p)
A) Zeff(2p) is larger in N than in O
B) Zeff(2p) is larger in O than in N
C) they are exactly the same
D) the ionization energy cannot be related to Zeff(2p)

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
25
What do you predict of the electron configuration of element 117?
A) [Rn]7s25f146d107p5
B) [Rn]7s26d107p6
C) [Rn]7s15f146d107p6
D) [Rn]7s27f147d107p5
E) [Rn]7s24f143d107p5
A) [Rn]7s25f146d107p5
B) [Rn]7s26d107p6
C) [Rn]7s15f146d107p6
D) [Rn]7s27f147d107p5
E) [Rn]7s24f143d107p5

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is always true?
A) IE1 increases with increasing atomic number
B) IE1 decreases with increasing atomic number
C) IE2 is always higher than IE1
D) for isoelectronic species the atom with the higher atomic number will have the lower IE1
E) none of the above are always true
A) IE1 increases with increasing atomic number
B) IE1 decreases with increasing atomic number
C) IE2 is always higher than IE1
D) for isoelectronic species the atom with the higher atomic number will have the lower IE1
E) none of the above are always true

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
27
Which Nobel gas has a full 4f shell of electrons?
A) Ne
B) Ar
C) Kr
D) Xe
E) Rn
A) Ne
B) Ar
C) Kr
D) Xe
E) Rn

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
28
The electron affinity for sodium is 52 kJ mol-1 and the first ionization energy for sodium is 496 kJ mol-1. What is the change in energy for the following reaction?
2 Na(g) → Na+(g) + Na-(g)
A) -548 kJ mol-1
B) -496 kJ mol-1
C) -52 kJ mol-1
D) +444 kJ mol-1
E) +888 kJ mol-1
2 Na(g) → Na+(g) + Na-(g)
A) -548 kJ mol-1
B) -496 kJ mol-1
C) -52 kJ mol-1
D) +444 kJ mol-1
E) +888 kJ mol-1

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
29
Which will have the largest electron affinity?
A) O
B) O-
C) O2-
D) O+
E) Ne
A) O
B) O-
C) O2-
D) O+
E) Ne

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
30
The wavefunction for the 2s orbital in hydrogen is given by 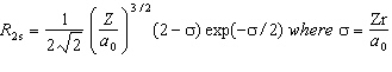 At what radius is there a node?
At what radius is there a node?
A) r = 0
B) r = 2/a0
C) r = a0
D) r = 2a0
E) there are no radial nodes in this function
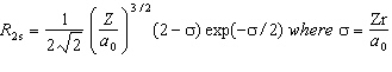 At what radius is there a node?
At what radius is there a node?A) r = 0
B) r = 2/a0
C) r = a0
D) r = 2a0
E) there are no radial nodes in this function

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
31
Which atomic species, in its ground state, has six unpaired electrons?
A) Fe
B) O
C) Mo
D) Cs
E) Dy
A) Fe
B) O
C) Mo
D) Cs
E) Dy

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
32
Of the following angular components for the wave functions of one-electron atoms, which one(s) must correspond only to s type orbitals?
A)
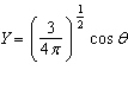
B)
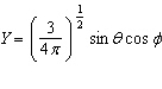
C)
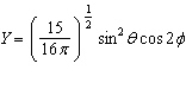
D)
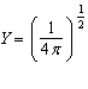
E) B and C
A)
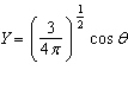
B)
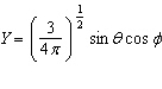
C)
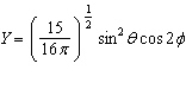
D)
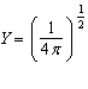
E) B and C

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck
33
Which species is diamagnetic?
A) N
B) Ne
C) O
D) A and B
E) None of the above
A) N
B) Ne
C) O
D) A and B
E) None of the above

Unlock Deck
Unlock for access to all 33 flashcards in this deck.
Unlock Deck
k this deck


