Deck 2: Elements of Life and Death: The Chemistry of Elements and Atoms
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/63
Play
Full screen (f)
Deck 2: Elements of Life and Death: The Chemistry of Elements and Atoms
1
What is the function of iron in our diet?
A) builds strong and healthy bones
B) boosts the immune system
C) supports proteins that promote growth of hair and nails
D) part of the protein that transports oxygen from the lungs to the tissues.
A) builds strong and healthy bones
B) boosts the immune system
C) supports proteins that promote growth of hair and nails
D) part of the protein that transports oxygen from the lungs to the tissues.
D
2
Which element can be found in the center of each heme ring that makes up the protein hemoglobin?
A) iron
B) calcium
C) helium
D) mercury
E) sodium
A) iron
B) calcium
C) helium
D) mercury
E) sodium
A
3
Which of the following is an extensive physical property?
A) temperature
B) density
C) mass
D) color e melting point
A) temperature
B) density
C) mass
D) color e melting point
C
4
What is the density of a block of metal with volume 5.25 cm3 and mass 14.18 g?
A) 0.37 g/cm3
B) 3.70 g/cm3
C) 19.43 g/cm3
D) 27.00 g/cm3
E) 2.70 g/cm3
A) 0.37 g/cm3
B) 3.70 g/cm3
C) 19.43 g/cm3
D) 27.00 g/cm3
E) 2.70 g/cm3

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
5
Choose the material that would sink in water (density of water = 1.00 g/cm3).
A) mass of 25.12 g; volume of 12.76 cm3
B) mass of 16.53 g; volume of 47.15 cm3
C) mass of 35.00 g; volume of 52.25 cm3
D) mass of 8.19 g; volume of 12.15 cm3
E) Mass of 115.00 g; volume of 224.35 cm3
A) mass of 25.12 g; volume of 12.76 cm3
B) mass of 16.53 g; volume of 47.15 cm3
C) mass of 35.00 g; volume of 52.25 cm3
D) mass of 8.19 g; volume of 12.15 cm3
E) Mass of 115.00 g; volume of 224.35 cm3

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
6
An astronaut weighs 120 pounds on earth. On the moon, where gravity is one-sixth that of the earth, she would weigh
A) 120 pounds.
B) 0 pounds.
C) 180 pounds.
D) 20 pounds.
A) 120 pounds.
B) 0 pounds.
C) 180 pounds.
D) 20 pounds.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
7
A cube has a side measuring 4 cm, and mass of 80 g The cube has a density of
A) 1.25 g/cm3 and would sink in water.
B) 1.25 g/cm3 and would float in water.
C) 0.80 g/cm3 and would sink in water.
D) 0.80 g/cm3 and would float in water.
E) 20.0 g/cm3 and would sink in water.
A) 1.25 g/cm3 and would sink in water.
B) 1.25 g/cm3 and would float in water.
C) 0.80 g/cm3 and would sink in water.
D) 0.80 g/cm3 and would float in water.
E) 20.0 g/cm3 and would sink in water.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
8
A box of silver-colored coins was found to contain coins having a mass of 3,458 g and, by water displacement, their volume was measured as 329.6 cm³. Given the following densities, identify what the coins are made of.
A) Platinum
B) Silver
C) Zinc
D) Tin
E) Aluminum
A) Platinum
B) Silver
C) Zinc
D) Tin
E) Aluminum

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is a chemical change?
A) Ice cubes melt when removed from the freezer.
B) Sugar dissolves in water.
C) Nail polish remover evaporates.
D) Silver tarnishes.
E) Eggs are whisked.
A) Ice cubes melt when removed from the freezer.
B) Sugar dissolves in water.
C) Nail polish remover evaporates.
D) Silver tarnishes.
E) Eggs are whisked.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is a physical change?
A) Food is digested.
B) Wood is burned in a stove.
C) Food color is dropped into water to give it color.
D) Fireworks explode.
E) A bicycle rusts.
A) Food is digested.
B) Wood is burned in a stove.
C) Food color is dropped into water to give it color.
D) Fireworks explode.
E) A bicycle rusts.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
11
Identify the homogeneous mixture.
A) an egg, before it is cracked
B) a bowl of cereal and milk
C) a cup of coffee
D) a bowl of M&Ms
E) a blueberry muffin
A) an egg, before it is cracked
B) a bowl of cereal and milk
C) a cup of coffee
D) a bowl of M&Ms
E) a blueberry muffin

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
12
Identify the heterogeneous mixture.
A) a container of mixed nuts
B) a bucket of seawater
C) a jar of mayonnaise
D) a gallon of milk
E) a gold bar
A) a container of mixed nuts
B) a bucket of seawater
C) a jar of mayonnaise
D) a gallon of milk
E) a gold bar

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following substances is/are elements?
I) Air II. Helium
III. Carbon monoxide
IV) Quartz
V) Chlorine
A) II only
B) II and V
C) I and V
D) I, III, and IV
E) IV only
I) Air II. Helium
III. Carbon monoxide
IV) Quartz
V) Chlorine
A) II only
B) II and V
C) I and V
D) I, III, and IV
E) IV only

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following substances is/are compounds?
I) Air
II) Helium
III) Carbon monoxide
IV) Quartz
V) Chlorine
A) III only
B) II and V
C) III and IV
D) I, III, and IV
E) IV only
I) Air
II) Helium
III) Carbon monoxide
IV) Quartz
V) Chlorine
A) III only
B) II and V
C) III and IV
D) I, III, and IV
E) IV only

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following substances is/are mixtures?
I) Air
II) Helium
III) Carbon monoxide
IV) Quartz
V) Chlorine
A) II only
B) II and V
C) I and V
D) I, III, and IV
E) I only
I) Air
II) Helium
III) Carbon monoxide
IV) Quartz
V) Chlorine
A) II only
B) II and V
C) I and V
D) I, III, and IV
E) I only

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
16
Choose the element symbol for the element in period 5, Group 4A.
A) As
B) Zr
C) Sb
D) Sn
E) Nb
A) As
B) Zr
C) Sb
D) Sn
E) Nb

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
17
Which element would you expect to have properties most similar to chlorine?
A) Sulfur, S
B) Astatine, At
C) Argon, Ar
D) Aluminum, Al
E) Sodium, Na
A) Sulfur, S
B) Astatine, At
C) Argon, Ar
D) Aluminum, Al
E) Sodium, Na

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
18
Choose the element symbol for the noble gas found in Period 4.
A) Br
B) Kr
C) K
D) Sc
E) Se
A) Br
B) Kr
C) K
D) Sc
E) Se

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
19
Elements in the periodic table are arranged according to their
A) atomic mass.
B) number of neutrons.
C) element symbol.
D) size.
E) atomic number.
A) atomic mass.
B) number of neutrons.
C) element symbol.
D) size.
E) atomic number.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
20
Most of the elements on the periodic table are
A) artificially made.
B) lanthanides.
C) non-metals.
D) noble gases.
E) metals.
A) artificially made.
B) lanthanides.
C) non-metals.
D) noble gases.
E) metals.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
21
Dmitri Mendeleev discovered periodicity in the elements by noticing what about elements that repeats at regular intervals?
A) ratios of atoms that combine to form compounds
B) metallic character
C) reactivity
D) a and c
E) b and c
A) ratios of atoms that combine to form compounds
B) metallic character
C) reactivity
D) a and c
E) b and c

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
22
Although human hair varies in diameter, an average width is 0.00075 m. Change this value to micrometers (μm). One meter = 1 106 μm.
A) 7.5 10-2 μm
B) 7.5 102 μm
C) 7.5 10-10 μm
D) 7.5 1010 μm
A) 7.5 10-2 μm
B) 7.5 102 μm
C) 7.5 10-10 μm
D) 7.5 1010 μm

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
23
An e. coli bacterium measures approximately 0.0000005 m in width. Change this value to nanometers (nm). One meter = 1 109 nm.
A) 5.0 10-16 nm
B) 5.0 1016 nm
C) 5.0 10-2 nm
D) 5.0 102 nm
A) 5.0 10-16 nm
B) 5.0 1016 nm
C) 5.0 10-2 nm
D) 5.0 102 nm

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
24
The distance from the Earth to the Sun is 1.49 1011 m. Change this value to kilometers (km). One km = 1 103 m.
A) 1.49 108 km
B) 1.49 1014 km
C) 1.49 107 km
D) 1.49 10-3 km
A) 1.49 108 km
B) 1.49 1014 km
C) 1.49 107 km
D) 1.49 10-3 km

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
25
Many bacteria can swim as fast as 50 μm per second. Calculate the distance, in meters, that a bacterium could swim in one day. One meter = 1 106 μm.
A) 5.0 10-4 m
B) 4.32 m
C) 4.32 106 m
D) 5.0 108 m
E) 1.8 10-1 m
A) 5.0 10-4 m
B) 4.32 m
C) 4.32 106 m
D) 5.0 108 m
E) 1.8 10-1 m

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
26
The average adult heart beats around 80 times per minute. How many heartbeats does the average adult have over a time span of 60 years?
A) 2.5 10-9 heartbeats
B) 4.2 107 heartbeats
C) 2.5 109 heartbeats
D) 4.2 10-7 heartbeats
A) 2.5 10-9 heartbeats
B) 4.2 107 heartbeats
C) 2.5 109 heartbeats
D) 4.2 10-7 heartbeats

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is the best description of the composition of an atom?
A) protons in the nucleus, electrons in a surrounding space
B) protons and neutrons in the nucleus, electrons in a surrounding space
C) neutrons and electrons in the nucleus, protons in a surrounding space
D) neutrons in the nucleus, protons and electrons in a surrounding space
E) protons and electrons in the nucleus, neutrons in a surrounding space
A) protons in the nucleus, electrons in a surrounding space
B) protons and neutrons in the nucleus, electrons in a surrounding space
C) neutrons and electrons in the nucleus, protons in a surrounding space
D) neutrons in the nucleus, protons and electrons in a surrounding space
E) protons and electrons in the nucleus, neutrons in a surrounding space

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
28
Choose the element symbol to replace X:
A) Cl
B) C
C) Br
D) Ar
E) F
A) Cl
B) C
C) Br
D) Ar
E) F

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
29
Choose the element symbol to replace X:
A) Si
B) N
C) O
D) F
E) Na
A) Si
B) N
C) O
D) F
E) Na

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
30
Isotopes of the same element have different numbers of
A) protons.
B) neutrons.
C) electrons.
D) atoms.
E) molecules.
A) protons.
B) neutrons.
C) electrons.
D) atoms.
E) molecules.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
31
How many protons and neutrons are found in Sodium-22?
A) 22 protons, 22 neutrons
B) 11 protons, 11 neutrons
C) 10 protons, 12 neutrons
D) 22 protons, 11 neutrons
E) 11 protons, 22 neutrons
A) 22 protons, 22 neutrons
B) 11 protons, 11 neutrons
C) 10 protons, 12 neutrons
D) 22 protons, 11 neutrons
E) 11 protons, 22 neutrons

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
32
How many protons and neutrons are found in Potassium-40?
A) 40 protons, 40 neutrons
B) 20 protons, 20 neutrons
C) 21 protons, 19 neutrons
D) 19 protons, 21 neutrons
E) 19 protons, 40 neutrons
A) 40 protons, 40 neutrons
B) 20 protons, 20 neutrons
C) 21 protons, 19 neutrons
D) 19 protons, 21 neutrons
E) 19 protons, 40 neutrons

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
33
How do we find the number of protons in an element?
A) Subtract the mass number from the atomic number.
B) Subtract the atomic number from the mass number.
C) mass number
D) atomic number
E) group number
A) Subtract the mass number from the atomic number.
B) Subtract the atomic number from the mass number.
C) mass number
D) atomic number
E) group number

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
34
How do we find the number of neutrons in an isotope?
A) Subtract the mass number from the atomic number.
B) Subtract the atomic number from the mass number.
C) atomic number
D) mass number
E) period number
A) Subtract the mass number from the atomic number.
B) Subtract the atomic number from the mass number.
C) atomic number
D) mass number
E) period number

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
35
An element has three naturally occurring isotopes, as described in the following table. Calculate the relative atomic mass and identify the element.
A) 20.18; neon
B) 20.99; neon
C) 20.17; calcium
D) 20.99; calcium
E) 31.48; sulfur
A) 20.18; neon
B) 20.99; neon
C) 20.17; calcium
D) 20.99; calcium
E) 31.48; sulfur

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
36
An element has two naturally occurring isotopes, as described in the following table. Calculate the relative atomic mass and identify the element.
A) 63.54; europium
B) 63.93; copper
C) 63.54; copper
D) 50.00; vanadium
E) 69.17; gallium
A) 63.54; europium
B) 63.93; copper
C) 63.54; copper
D) 50.00; vanadium
E) 69.17; gallium

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
37
Choose the element with 5 valence electrons.
A) cesium
B) vanadium
C) zirconium
D) boron
E) phosphorus
A) cesium
B) vanadium
C) zirconium
D) boron
E) phosphorus

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
38
Give the number of valence electrons, and the total number of electrons for chlorine.
A) 7 valence; 17 total
B) 5 valence; 17 total
C) 3 valence; 17 total
D) 7 valence; 8 total
E) 5 valence; 8 total
A) 7 valence; 17 total
B) 5 valence; 17 total
C) 3 valence; 17 total
D) 7 valence; 8 total
E) 5 valence; 8 total

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
39
Give the number of valence electrons, and the total number of electrons for silicon.
A) 14 valence; 14 total
B) 4 valence; 14 total
C) 3 valence; 14 total
D) 4 valence; 8 total
E) 3 valence; 8 total
A) 14 valence; 14 total
B) 4 valence; 14 total
C) 3 valence; 14 total
D) 4 valence; 8 total
E) 3 valence; 8 total

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
40
Give the number of protons and electrons for chloride, the ion formed from chlorine.
A) 18 protons; 17 electrons
B) 17 protons; 19 electrons
C) 17 protons; 17 electrons
D) 17 protons; 18 electrons
E) 18 protons; 16 electrons
A) 18 protons; 17 electrons
B) 17 protons; 19 electrons
C) 17 protons; 17 electrons
D) 17 protons; 18 electrons
E) 18 protons; 16 electrons

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
41
Give the number of protons and electrons for the ion formed from calcium.
A) 20 protons; 18 electrons
B) 18 protons; 20 electrons
C) 20 protons; 19 electrons
D) 18 protons; 19 electrons
E) 22 protons; 20 electrons
A) 20 protons; 18 electrons
B) 18 protons; 20 electrons
C) 20 protons; 19 electrons
D) 18 protons; 19 electrons
E) 22 protons; 20 electrons

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
42
Choose the element that would form an ion with a charge of -2.
A) Sr
B) Na
C) Se
D) Ne
E) Br
A) Sr
B) Na
C) Se
D) Ne
E) Br

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
43
Choose the element that would form an ion with a charge of +1.
A) F
B) He
C) As
D) K
E) Be
A) F
B) He
C) As
D) K
E) Be

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
44
Choose the best definition of a quantum mechanical orbital.
A) a circular path that the electron follows around the nucleus
B) the space around the nucleus where the protons may be found
C) region of space where there is a high probability of finding an electron
D) the energy level that an atom's neutrons occupy
E) region of space where electrons feel the strongest "pull" from the nucleus
A) a circular path that the electron follows around the nucleus
B) the space around the nucleus where the protons may be found
C) region of space where there is a high probability of finding an electron
D) the energy level that an atom's neutrons occupy
E) region of space where electrons feel the strongest "pull" from the nucleus

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
45
The element that plays an important role in forming healthy bones and teeth is
A) iron.
B) calcium.
C) arsenic.
D) magnesium.
E) phosphorus.
A) iron.
B) calcium.
C) arsenic.
D) magnesium.
E) phosphorus.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
46
The risk of contracting this disorder can be reduced by consuming enough calcium at an early age.
A) anemia
B) osmosis
C) tooth decay
D) ornithosis
E) osteoporosis
A) anemia
B) osmosis
C) tooth decay
D) ornithosis
E) osteoporosis

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
47
This element is poisonous because of its similarity to an essential element for life.
A) arsenic
B) silicon
C) phosphorus
D) sulfur
E) chlorine
A) arsenic
B) silicon
C) phosphorus
D) sulfur
E) chlorine

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
48
Which of these is not one of the six building block elements used to make biological molecules?
A) carbon
B) hydrogen
C) phosphorus
D) magnesium
E) sulfur
A) carbon
B) hydrogen
C) phosphorus
D) magnesium
E) sulfur

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
49
The condition caused by low levels of iron in the body is called _________________________.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
50
Explain the difference between mass and weight.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
51
Use a periodic table and fill in the blanks in the following chart below:
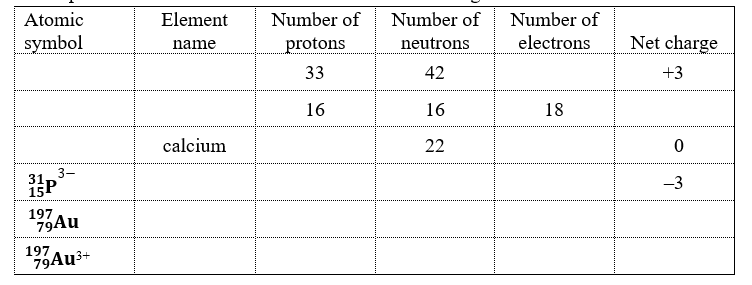


Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
52
Explain the difference between the mass number, the atomic number, and the relative atomic mass.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
53
Elements with the same number of _______________________ ______________________ have the similar chemical properties.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
54
Give the number of total electrons and valence electrons for the element strontium, Sr.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
55
When the element strontium (Sr) ionizes, would you expect it to gain or lose electrons? How many?

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
56
When elements ionize, they gain or lose electrons to resemble the configuration of the nearest ______________________.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
57
Quantum mechanics describes electrons not as particles but as ____________________.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
58
Match each of the following elements to its description.
-Mercury (Hg)
A)This element has 5 valence electrons and is a poison that can be found in drinking water.
B)Having 8 protons, this element is essential for life.
C)One of the only elements to exist as a liquid at room temperature, this is a dangerous environmental toxin that accumulates in fish.
D)This element loses 2 electrons to form a +2 ion; not consuming enough of it can increase risk of osteoporosis.
E)This element gains three electrons when forming an ion; it also helps form an essential source of energy for cells.
F)This is one of the transition elements, and is essential for the function of the protein hemoglobin.
-Mercury (Hg)
A)This element has 5 valence electrons and is a poison that can be found in drinking water.
B)Having 8 protons, this element is essential for life.
C)One of the only elements to exist as a liquid at room temperature, this is a dangerous environmental toxin that accumulates in fish.
D)This element loses 2 electrons to form a +2 ion; not consuming enough of it can increase risk of osteoporosis.
E)This element gains three electrons when forming an ion; it also helps form an essential source of energy for cells.
F)This is one of the transition elements, and is essential for the function of the protein hemoglobin.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
59
Match each of the following elements to its description.
-Phosphorus (P)
A)This element has 5 valence electrons and is a poison that can be found in drinking water.
B)Having 8 protons, this element is essential for life.
C)One of the only elements to exist as a liquid at room temperature, this is a dangerous environmental toxin that accumulates in fish.
D)This element loses 2 electrons to form a +3 ion; not consuming enough of it can increase risk of osteoporosis.
E)This element gains three electrons when forming an ion; it also helps form an essential source of energy for cells.
F)This is one of the transition elements, and is essential for the function of the protein hemoglobin.
-Phosphorus (P)
A)This element has 5 valence electrons and is a poison that can be found in drinking water.
B)Having 8 protons, this element is essential for life.
C)One of the only elements to exist as a liquid at room temperature, this is a dangerous environmental toxin that accumulates in fish.
D)This element loses 2 electrons to form a +3 ion; not consuming enough of it can increase risk of osteoporosis.
E)This element gains three electrons when forming an ion; it also helps form an essential source of energy for cells.
F)This is one of the transition elements, and is essential for the function of the protein hemoglobin.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
60
Match each of the following elements to its description.
-Arsenic (As)
A)This element has 5 valence electrons and is a poison that can be found in drinking water.
B)Having 8 protons, this element is essential for life.
C)One of the only elements to exist as a liquid at room temperature, this is a dangerous environmental toxin that accumulates in fish.
D)This element loses 2 electrons to form a +4 ion; not consuming enough of it can increase risk of osteoporosis.
E)This element gains three electrons when forming an ion; it also helps form an essential source of energy for cells.
F)This is one of the transition elements, and is essential for the function of the protein hemoglobin.
-Arsenic (As)
A)This element has 5 valence electrons and is a poison that can be found in drinking water.
B)Having 8 protons, this element is essential for life.
C)One of the only elements to exist as a liquid at room temperature, this is a dangerous environmental toxin that accumulates in fish.
D)This element loses 2 electrons to form a +4 ion; not consuming enough of it can increase risk of osteoporosis.
E)This element gains three electrons when forming an ion; it also helps form an essential source of energy for cells.
F)This is one of the transition elements, and is essential for the function of the protein hemoglobin.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
61
Match each of the following elements to its description.
-Iron (Fe)
A)This element has 5 valence electrons and is a poison that can be found in drinking water.
B)Having 8 protons, this element is essential for life.
C)One of the only elements to exist as a liquid at room temperature, this is a dangerous environmental toxin that accumulates in fish.
D)This element loses 2 electrons to form a +5 ion; not consuming enough of it can increase risk of osteoporosis.
E)This element gains three electrons when forming an ion; it also helps form an essential source of energy for cells.
F)This is one of the transition elements, and is essential for the function of the protein hemoglobin.
-Iron (Fe)
A)This element has 5 valence electrons and is a poison that can be found in drinking water.
B)Having 8 protons, this element is essential for life.
C)One of the only elements to exist as a liquid at room temperature, this is a dangerous environmental toxin that accumulates in fish.
D)This element loses 2 electrons to form a +5 ion; not consuming enough of it can increase risk of osteoporosis.
E)This element gains three electrons when forming an ion; it also helps form an essential source of energy for cells.
F)This is one of the transition elements, and is essential for the function of the protein hemoglobin.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
62
Match each of the following elements to its description.
-Calcium (Ca)
A)This element has 5 valence electrons and is a poison that can be found in drinking water.
B)Having 8 protons, this element is essential for life.
C)One of the only elements to exist as a liquid at room temperature, this is a dangerous environmental toxin that accumulates in fish.
D)This element loses 2 electrons to form a +6 ion; not consuming enough of it can increase risk of osteoporosis.
E)This element gains three electrons when forming an ion; it also helps form an essential source of energy for cells.
F)This is one of the transition elements, and is essential for the function of the protein hemoglobin.
-Calcium (Ca)
A)This element has 5 valence electrons and is a poison that can be found in drinking water.
B)Having 8 protons, this element is essential for life.
C)One of the only elements to exist as a liquid at room temperature, this is a dangerous environmental toxin that accumulates in fish.
D)This element loses 2 electrons to form a +6 ion; not consuming enough of it can increase risk of osteoporosis.
E)This element gains three electrons when forming an ion; it also helps form an essential source of energy for cells.
F)This is one of the transition elements, and is essential for the function of the protein hemoglobin.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
63
Match each of the following elements to its description.
-Oxygen (O)
A)This element has 5 valence electrons and is a poison that can be found in drinking water.
B)Having 8 protons, this element is essential for life.
C)One of the only elements to exist as a liquid at room temperature, this is a dangerous environmental toxin that accumulates in fish.
D)This element loses 2 electrons to form a +7 ion; not consuming enough of it can increase risk of osteoporosis.
E)This element gains three electrons when forming an ion; it also helps form an essential source of energy for cells.
F)This is one of the transition elements, and is essential for the function of the protein hemoglobin.
-Oxygen (O)
A)This element has 5 valence electrons and is a poison that can be found in drinking water.
B)Having 8 protons, this element is essential for life.
C)One of the only elements to exist as a liquid at room temperature, this is a dangerous environmental toxin that accumulates in fish.
D)This element loses 2 electrons to form a +7 ion; not consuming enough of it can increase risk of osteoporosis.
E)This element gains three electrons when forming an ion; it also helps form an essential source of energy for cells.
F)This is one of the transition elements, and is essential for the function of the protein hemoglobin.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck


