Deck 26: S-Block Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/28
Play
Full screen (f)
Deck 26: S-Block Chemistry
1
The properties of the Group 1 metals include? Please select all that apply.
A) Distinctive visible spectra
B) High melting points
C) Easy oxidation to M+
D) Reaction with water evolves hydrogen
A) Distinctive visible spectra
B) High melting points
C) Easy oxidation to M+
D) Reaction with water evolves hydrogen
A, C, D
2
The Down's cell is used to produce sodium metal by electrolysis of molten sodium chloride.
True
3
The lattice enthalpy as determined by the Kapustinskii equation of K2O is larger in magnitude than the lattice enthalpy of K2O2.
True
4
In the suboxide Cs11O3 , the electrons are delocalized over the whole structure. Give a formula that more adequately represents this arrangement.
A) (Cs+)11(O-)3(e-)8
B) (Cs+)11(O)3(e-)11
C) (Cs+)6(Cs)5(O2-)3
D) (Cs+)11(O2-)3(e-)5
A) (Cs+)11(O-)3(e-)8
B) (Cs+)11(O)3(e-)11
C) (Cs+)6(Cs)5(O2-)3
D) (Cs+)11(O2-)3(e-)5

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
5
Sodium hydroxide is prepared industrially by electrolysis of a solution of sodium chloride.

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
6
The reaction of lithium with water is much slower than that of caesium, but the hydration enthalpy is higher because. Please select all that apply.
A) Cs reacts with water to form an insoluble oxide not a soluble hydroxide.
B) Lithium has a much larger charge to radius ratio than caesium and attracts more water molecules on hydration
C) Caesium has a much larger charge to radius ratio than lithium and attracts more water molecules on hydration
D) The heat of the reaction is enough to melt the caesium metal as it has a low melting point.
A) Cs reacts with water to form an insoluble oxide not a soluble hydroxide.
B) Lithium has a much larger charge to radius ratio than caesium and attracts more water molecules on hydration
C) Caesium has a much larger charge to radius ratio than lithium and attracts more water molecules on hydration
D) The heat of the reaction is enough to melt the caesium metal as it has a low melting point.

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
7
Which of these enthalpy cycles correctly accounts for the formation of a metal carbonate from its ions
A)
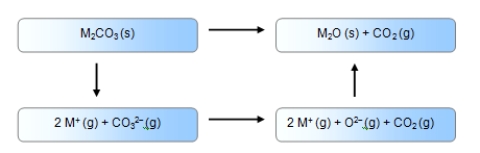 Alt text needed
Alt text needed
B)
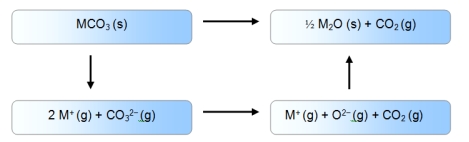 Alt text needed
Alt text needed
C)
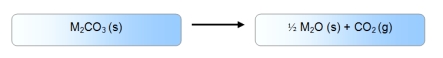 Alt text needed
Alt text needed
D)
 Alt text needed
Alt text needed
A)
 Alt text needed
Alt text neededB)
 Alt text needed
Alt text neededC)
 Alt text needed
Alt text neededD)
 Alt text needed
Alt text needed
Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
8
Match the reaction products with their reactants.
-sodium + oxygen
A) sodium peroxide
B) sodium hydroxide plus hydrogen
C) sodium oxide plus oxygen
D) sodium amide plus hydrogen
-sodium + oxygen
A) sodium peroxide
B) sodium hydroxide plus hydrogen
C) sodium oxide plus oxygen
D) sodium amide plus hydrogen

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
9
Match the reaction products with their reactants.
-sodium + water
A) sodium peroxide
B) sodium hydroxide plus hydrogen
C) sodium oxide plus oxygen
D) sodium amide plus hydrogen
-sodium + water
A) sodium peroxide
B) sodium hydroxide plus hydrogen
C) sodium oxide plus oxygen
D) sodium amide plus hydrogen

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
10
Match the reaction products with their reactants.
-heat + sodium peroxide
A) sodium peroxide
B) sodium hydroxide plus hydrogen
C) sodium oxide plus oxygen
D) sodium amide plus hydrogen
-heat + sodium peroxide
A) sodium peroxide
B) sodium hydroxide plus hydrogen
C) sodium oxide plus oxygen
D) sodium amide plus hydrogen

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
11
Match the reaction products with their reactants.
-sodium + ammonia
A) sodium peroxide
B) sodium hydroxide plus hydrogen
C) sodium oxide plus oxygen
D) sodium amide plus hydrogen
-sodium + ammonia
A) sodium peroxide
B) sodium hydroxide plus hydrogen
C) sodium oxide plus oxygen
D) sodium amide plus hydrogen

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
12
While Group 1 metal cations generally coordinate weakly to most ligands, they do form stable complexes with size selective macrocyclic ligands.

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
13
The properties of beryllium are markedly different from the rest of the Group 2 elements. Why? Please select all that apply.
A) Beryllium has a high charge ratio.
B) Beryllium shows much covalent character in its bonding.
C) Beryllium is highly unstable and no compounds have been isolated.
D) Beryllium reacts with water to produce Be(OH)2.
A) Beryllium has a high charge ratio.
B) Beryllium shows much covalent character in its bonding.
C) Beryllium is highly unstable and no compounds have been isolated.
D) Beryllium reacts with water to produce Be(OH)2.

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
14
The concentration of hydroxide ions in a saturated solution of Ca(OH)2 is 4.18 x 10-3 mol dm-3. What is the pH of this solution?
A) 5.6
B) 11.6
C) 12.2
D) 1.8
A) 5.6
B) 11.6
C) 12.2
D) 1.8

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
15
Beryllium hydroxide, like aluminium hydroxide, shows ____________ behaviour.

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
16
Magnesium hydroxide is widely used in the indigestion remedy, milk of magnesia. Barium hydroxide would be a better antacid than magnesium hydroxide.

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
17
Neither the Born Lande or the Kapustinskii equations correctly calculate the lattice energy for beryllium compounds. Why?
A) The Born Lande and Kapustinskii models are based on ionic bonding and the bonding in beryllium compounds is largely covalent.
B) The ionic radius of beryllium is an estimate as no beryllium compounds have been isolated.
C) Beryllium compounds do not form the usual lattice types and no value of the Madelung constant for their structures have been calculated.
D) The first ionisation potential of beryllium is not known.
A) The Born Lande and Kapustinskii models are based on ionic bonding and the bonding in beryllium compounds is largely covalent.
B) The ionic radius of beryllium is an estimate as no beryllium compounds have been isolated.
C) Beryllium compounds do not form the usual lattice types and no value of the Madelung constant for their structures have been calculated.
D) The first ionisation potential of beryllium is not known.

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
18
The Kapustinskii equation predicts that the lattice enthalpy increases from MCl to MCl2 to MCl3.

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
19
Quicklime can be used in bricklaying to produce a strong mortar after a few days of hardening in air. During the hardening process Ca(OH)2 reacts with ____ from the air.

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
20
A trainee technician is asked to prepare solutions of calcium hydroxide and barium hydroxide for a laboratory class the following week. Just as he has finished weighing out the solid samples of CaO and BaO, the fire alarm is tested and as it is the end of the day, the technician goes home. Next morning as he is trying to dissolve the BaO sample it will not dissolve in water. Why might this be?
A) The alkali metal oxides are insoluble in water.
B) Barium oxide is light sensitive and has decomposed to insoluble products.
C) BaO is not stable in air as in the presence of carbon dioxide, barium oxide has transformed to the insoluble barium carbonate.
D) BaO rapidly reacts with oxygen in the air to form BaO2 which is insoluble in water.
A) The alkali metal oxides are insoluble in water.
B) Barium oxide is light sensitive and has decomposed to insoluble products.
C) BaO is not stable in air as in the presence of carbon dioxide, barium oxide has transformed to the insoluble barium carbonate.
D) BaO rapidly reacts with oxygen in the air to form BaO2 which is insoluble in water.

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
21
White vinegar is a cheap alternative to the many expensive limescale removers on the market. Which equation correctly describes the reaction between limescale and vinegar?
A) CaCO3 (s) + 2 CH3CO2H (aq) Ca(CH3CO2)2 (aq) + H2O (l) + CO2 (g)
B) CaCO3 (s) + 2 CO2H2 (aq) Ca(HCOO)2 (aq) + H2O (l) + CO2 (g)
C) CaCO3 (s) + CH3CO2H (aq) Ca(CH2CO2)2 (aq) + H2O (l) + CO2 (g)
D) CaCO3 (s) + CO2H2 (aq) Ca(COO) (aq) + H2O (l) + CO2 (g)
A) CaCO3 (s) + 2 CH3CO2H (aq) Ca(CH3CO2)2 (aq) + H2O (l) + CO2 (g)
B) CaCO3 (s) + 2 CO2H2 (aq) Ca(HCOO)2 (aq) + H2O (l) + CO2 (g)
C) CaCO3 (s) + CH3CO2H (aq) Ca(CH2CO2)2 (aq) + H2O (l) + CO2 (g)
D) CaCO3 (s) + CO2H2 (aq) Ca(COO) (aq) + H2O (l) + CO2 (g)

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
22
Compounds of beryllium form _____ solutions.

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
23
The coordination chemistry of the Group 2 cations is much greater than that of the Group 1 cations. Why?
A) The alkaline earth metal ions have a greater charge density.
B) The alkaline earth metals form more soluble compounds.
C) The alkaline earth metals are more reactive.
D) The alkali metal ions are too small to coordinate to many ligands.
A) The alkaline earth metal ions have a greater charge density.
B) The alkaline earth metals form more soluble compounds.
C) The alkaline earth metals are more reactive.
D) The alkali metal ions are too small to coordinate to many ligands.

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
24
Beryllium normally has a coordination number of _ in its compounds.

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
25
Beryllium chemistry is very different to the rest of the group. Which of the following statements apply to beryllium and not the rest of Group 2. Please select all that apply.
A) Beryllium hydroxide is amphoteric.
B) Beryllium oxide does not react with water to form beryllium hydroxide.
C) Beryllium forms many covalent compounds.
D) Beryllium does not form a divalent cation.
A) Beryllium hydroxide is amphoteric.
B) Beryllium oxide does not react with water to form beryllium hydroxide.
C) Beryllium forms many covalent compounds.
D) Beryllium does not form a divalent cation.

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
26
Methyl lithium exists as a
A) Monomer
B) Trimer
C) Dimer
D) Tetramer
A) Monomer
B) Trimer
C) Dimer
D) Tetramer

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
27
The compound EtMgBr is used extensively in organic chemistry as a _______ reagent.

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
28
Many metals form metallocene complexes where a metal ion is sandwiched between two aromatic rings. The ion is considered to be equally coordinated to all carbons in the ring.
For example cyclopentadience (C5H6) reacts with sodium to form the ionic compound (C5H5)- Na+. The ring structure of the ligand is aromatic and the structure drawn as delocalised. This ionic salt is then used to produce a host of coordination complexes e.g with Fe, Mg, Be etc which consist of a divalent cation and two cycolpentadienyl rings. Most of these ions form a sandwich complex where the metal ion lies equidistantly between the two rings. However, in the gas phase, beryllium coordinates to one ring or the other but not both. By considering the properties of beryllium, suggest why this might be.
A) Beryllium is so small that it brings the two negatively charged rings too close to each other and they repel. Be can therefore only coordinate to one ring at a time.
B) The rings are too bulky and can't get close to the beryllium as it is so small.
C) Beryllium must be neutral in this compound.
D) The orbitals of the beryllium hybridize to form four covalent bonds rather than the sandwich structure.
For example cyclopentadience (C5H6) reacts with sodium to form the ionic compound (C5H5)- Na+. The ring structure of the ligand is aromatic and the structure drawn as delocalised. This ionic salt is then used to produce a host of coordination complexes e.g with Fe, Mg, Be etc which consist of a divalent cation and two cycolpentadienyl rings. Most of these ions form a sandwich complex where the metal ion lies equidistantly between the two rings. However, in the gas phase, beryllium coordinates to one ring or the other but not both. By considering the properties of beryllium, suggest why this might be.
A) Beryllium is so small that it brings the two negatively charged rings too close to each other and they repel. Be can therefore only coordinate to one ring at a time.
B) The rings are too bulky and can't get close to the beryllium as it is so small.
C) Beryllium must be neutral in this compound.
D) The orbitals of the beryllium hybridize to form four covalent bonds rather than the sandwich structure.

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck


