Deck 24: Carboxylic Acids and Derivatives: Nucleophilic Acyl Substitution and Α-Substitution Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/25
Play
Full screen (f)
Deck 24: Carboxylic Acids and Derivatives: Nucleophilic Acyl Substitution and Α-Substitution Reactions
1
Rank the following molecules, A-D, in order of their relative electrophilicity (where 1 is the most electrophilic and 4 is the least electrophilic):
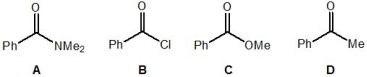
A) Molecule A
B) Molecule B
C) Molecule C
D) Molecule D

A) Molecule A
B) Molecule B
C) Molecule C
D) Molecule D
B, D, C, A
2
Rank the following molecules, A-D, in order of their relative acidity (where 1 is the most acidic and 4 is the least acidic):
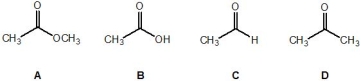
A) Molecule A
B) Molecule B
C) Molecule C
D) Molecule D

A) Molecule A
B) Molecule B
C) Molecule C
D) Molecule D
B, C, D, A
3
For the following reaction, pick out a suitable rate profile from A-D for this transformation:
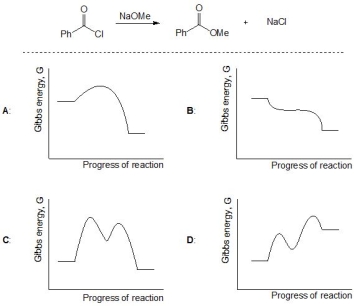
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D
C
4
For the following reaction, pick out the products. Please select all that apply.
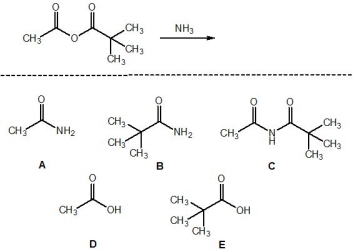
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
5
From the following reactions, A-D, pick out those which lead to the formation of ethyl acetate (ethyl ethanoate). Please select all that apply.
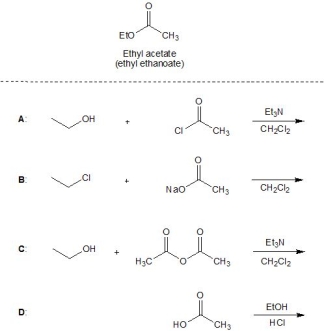
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
6
For the following transformation, deduce whether it is an oxidation, reduction or neither:

A) Oxidation
B) Reduction
C) Neither

A) Oxidation
B) Reduction
C) Neither

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
7
Rank the following molecules, A-D, in order of their ability to form enols (with 1 being most able and 4 the least able):
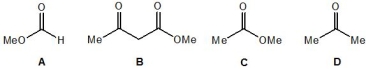
A) Molecule A
B) Molecule B
C) Molecule C
D) Molecule D

A) Molecule A
B) Molecule B
C) Molecule C
D) Molecule D

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
8
For the following reaction, pick out the major product:
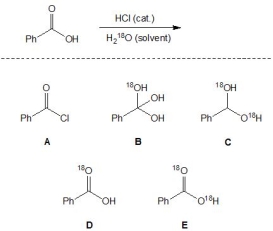
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
9
For the following reaction, pick out the product:
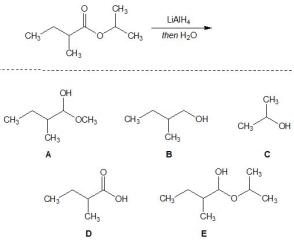
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
10
For the following reaction, pick out the major product::
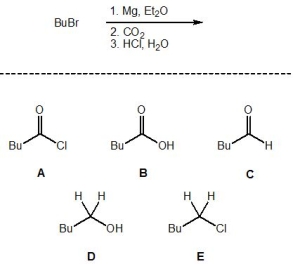
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
11
For the following reactions, A-D, pick out those which lead to the formation of 2-methyl-2-phenylethanol. Please select all that apply.
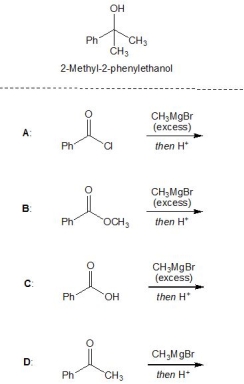
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
12
For the following reaction, pick out the major product:
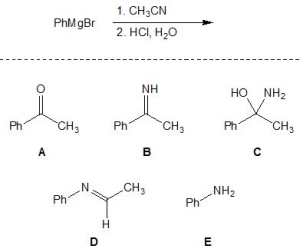
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following reagents, A-E, are suitable reagents for the following transformation? Please select all that apply.
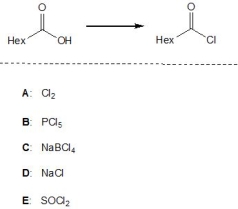
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following reagents, A-D, is a suitable reagent for the following transformation?
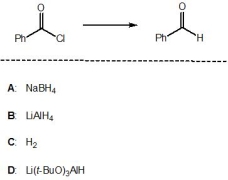
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following reagents, A-E, is a suitable reagent for the following transformation?
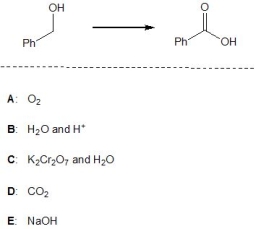
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following reagents, A-E, is a suitable reagent for the following transformation?
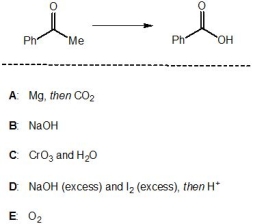
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
17
From the following equilibrium, pick out the approximate equilibrium constant for this process:

A) 10-0.64
B) 10-1.56
C) 10-9
D) 10-12
E) 10-16

A) 10-0.64
B) 10-1.56
C) 10-9
D) 10-12
E) 10-16

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
18
Rank the following molecules, A-D, in order of their relative acidity (where 1 is the most acidic and 4 is the least acidic):
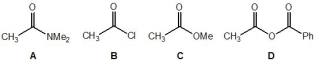
A) Molecule A
B) Molecule B
C) Molecule C
D) Molecule D

A) Molecule A
B) Molecule B
C) Molecule C
D) Molecule D

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
19
For the following Claisen reaction, pick out the product:
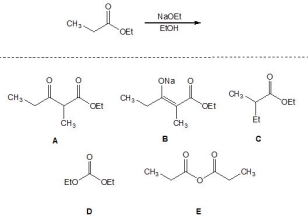
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
20
For the following reaction, pick out the major product:
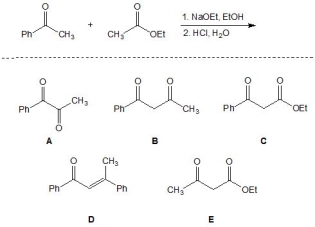
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
21
Identify the reaction class for the following reaction:
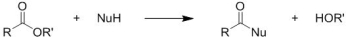
A) Nucleophilic acyl substitution
B) α-substitution
C) carbonyl-carbonyl condensation
D) nucleophilic alkyl substitution

A) Nucleophilic acyl substitution
B) α-substitution
C) carbonyl-carbonyl condensation
D) nucleophilic alkyl substitution

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
22
A Dieckmann reaction is an example of what type of process? Please select all that apply.
A) Saponification reaction
B) Claisen condensation reaction
C) An intramolecular reaction
D) SN2 reaction
A) Saponification reaction
B) Claisen condensation reaction
C) An intramolecular reaction
D) SN2 reaction

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
23
How can the formation of ethyl butanoate from butanoic acid and ethanol be favoured? Please select all that apply.
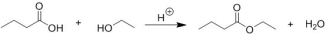
A) Add a drying agent (e.g. molecular sieves)
B) Distillation (e.g. using a Dean-Stark trap)
C) Lower reaction temperature (e.g. ice bath)
D) Use water as a solvent
E) Use ethanol as a solvent

A) Add a drying agent (e.g. molecular sieves)
B) Distillation (e.g. using a Dean-Stark trap)
C) Lower reaction temperature (e.g. ice bath)
D) Use water as a solvent
E) Use ethanol as a solvent

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
24
Predict the major product of the following reaction, at room temperature.
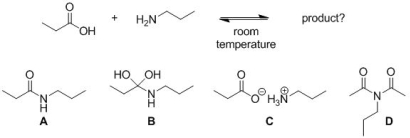
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
25
Identify the major enol of methyl 3-oxopentanoate.
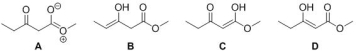
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck


