Deck 19: Organic Reaction Mechanisms
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/28
Play
Full screen (f)
Deck 19: Organic Reaction Mechanisms
1
From the following molecules, pick out the one which is the strongest nucleophile:
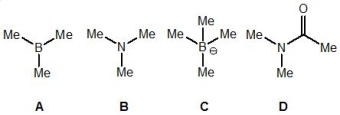
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D
B
2
Which of the following shows resonance?
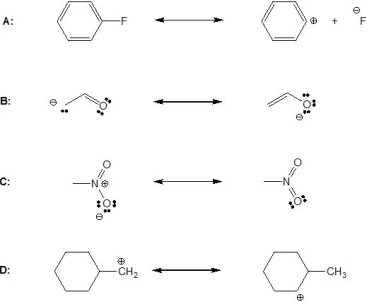
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D
B
3
Which of the following molecules contain conjugation? Please select all that apply.
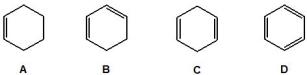
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D
B, D
4
Which of the following molecules exhibit conjugation? Please select all that apply.
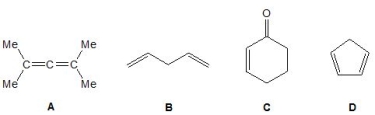
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following structures do not contribute to the resonance hybrid of a benzyl cation? Please select all that apply.
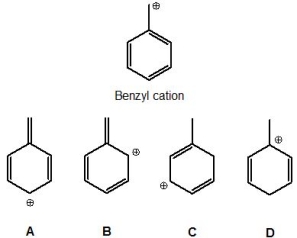
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
6
From the following carbocations, pick out the most stable:
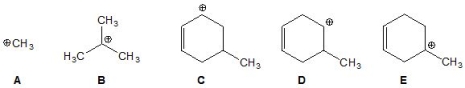
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
7
Match the following acids to their acidity constant, Ka (in water):
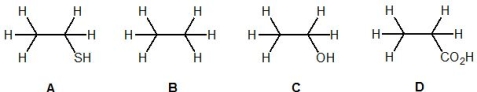
-Ka = 10-5
A) D
B) A
C) C
D) B

-Ka = 10-5
A) D
B) A
C) C
D) B

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
8
Match the following acids to their acidity constant, Ka (in water):

-Ka = 10-7
A) D
B) A
C) C
D) B

-Ka = 10-7
A) D
B) A
C) C
D) B

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
9
Match the following acids to their acidity constant, Ka (in water):

-Ka = 10-16
A) D
B) A
C) C
D) B

-Ka = 10-16
A) D
B) A
C) C
D) B

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
10
Match the following acids to their acidity constant, Ka (in water):

-Ka = 10-50
A) D
B) A
C) C
D) B

-Ka = 10-50
A) D
B) A
C) C
D) B

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
11
Using the pKa data provided, pick out the acid that is most dissociated in water.
A) Acid A; pKa = ?9
B) Acid B; pKa = 0
C) Acid C; pKa = +2
D) Acid D; pKa = +50
A) Acid A; pKa = ?9
B) Acid B; pKa = 0
C) Acid C; pKa = +2
D) Acid D; pKa = +50

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following phenols has the largest acidity constant, Ka?
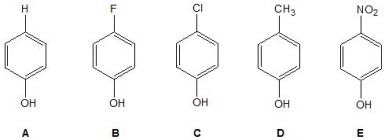
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
13
Toluene can be deprotonated using n-BuLi in THF; pick out its conjugate base:
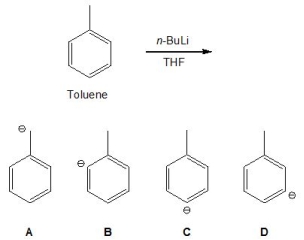
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
14
From the following molecules, pick out the strongest base:
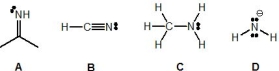
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
15
From the following molecules, pick out the strongest acid:
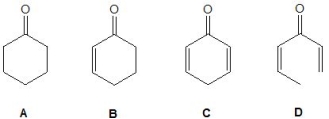
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
16
From the following molecules, pick out the strongest base:
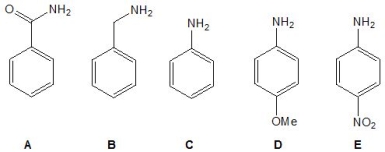
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
17
From the following molecules, pick out the weakest base:
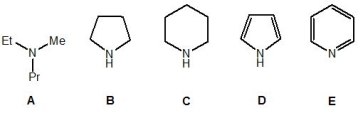
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
18
From the following carbocations, pick out the most acidic:
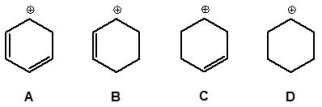
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
19
For the following reaction, deduce whether it involves oxidation, reduction or neither:

A) Oxidation
B) Reduction
C) Neither

A) Oxidation
B) Reduction
C) Neither

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
20
Pick out the term which describes the following reaction:
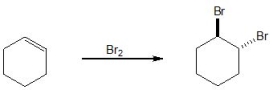
A) Radical elimination
B) Nucleophilic substitution
C) Electrophilic addition

A) Radical elimination
B) Nucleophilic substitution
C) Electrophilic addition

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
21
Pick out the term which describes the following reaction:
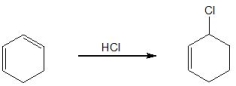
A) Chemoselectivity
B) Stereoselectivity
C) Regioselectivity

A) Chemoselectivity
B) Stereoselectivity
C) Regioselectivity

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
22
Pick out the terms which describe the following reaction. Please select all that apply.
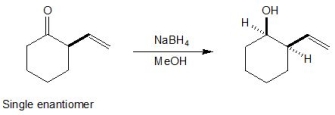
A) Chemoselectivity
B) Regioselectivity
C) Stereoselectivity

A) Chemoselectivity
B) Regioselectivity
C) Stereoselectivity

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
23
Pick out the terms which describe the following reaction. Please select all that apply.
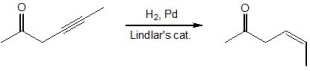
A) Chemoselectivity
B) Regioselectivity
C) Stereoselectivity

A) Chemoselectivity
B) Regioselectivity
C) Stereoselectivity

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
24
In general, the more stable the carbanion or carbocation, the higher the equilibrium constant (K) and the greater the concentration of the product carbanion or carbocation at equilibrium.

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
25
Select those from A-D that are electron donating groups through mesomeric effects (also known as resonance effects).
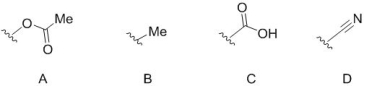
A) A
B) B
C) C
D) D
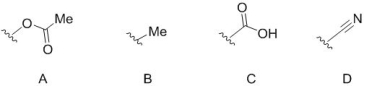
A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
26
Select molecules below that have conjugated double bonds (select all correct answers)
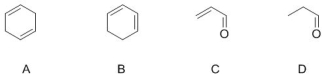
A) B
B) C
C) D
D) A

A) B
B) C
C) D
D) A

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
27
An electronegative atom (e.g. F) bonded to a carbon chain causes a polarization of the C-C bonds. The notation "- I" is often used to represent this, but to what does this refer?
A) negative inductive effect
B) positive induction effect
C) hyperconjugation
D) resonance
A) negative inductive effect
B) positive induction effect
C) hyperconjugation
D) resonance

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
28
The symmetrical cleavage of a covalent bond is called heterolytic cleavage.

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck


