Deck 12: Molecular Characterisation
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/30
Play
Full screen (f)
Deck 12: Molecular Characterisation
1
By elemental analysis, the carbon content of an unknown compound was found to be 85.7%. From the following molecular formulae, (a)-(d), pick out the most likely formula for this unknown compound:
A) C4H8O
B) C5H8O
C) C6H12
D) C6H6
A) C4H8O
B) C5H8O
C) C6H12
D) C6H6
C
2
Pick out the isotopic distribution pattern for 2-phenylpropanoic acid (hint: 1.1% of all carbon atoms have an atomic mass of 13).
A) 150 (M, 100%), 151 (1%), 152 (0.01%).
B) 150 (M, 100%), 151 (5%), 152 (0.05%).
C) 150 (M, 100%), 151 (10%), 152 (0.1%).
D) 150 (M, 100%), 151 (20%), 152 (0.2%).
A) 150 (M, 100%), 151 (1%), 152 (0.01%).
B) 150 (M, 100%), 151 (5%), 152 (0.05%).
C) 150 (M, 100%), 151 (10%), 152 (0.1%).
D) 150 (M, 100%), 151 (20%), 152 (0.2%).
C
3
Pick out the molecular ion peak for a molecule containing a single nitrogen atom.
A) 84.
B) 93.
C) 108.
D) 114.
A) 84.
B) 93.
C) 108.
D) 114.
B
4
Match the following molecular formulae to the mass spectra A-C:
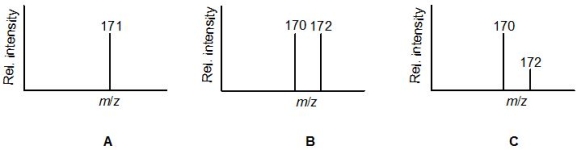
-C7H7Br
A) B
B) C
C) A

-C7H7Br
A) B
B) C
C) A

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
5
Match the following molecular formulae to the mass spectra A-C:

-C8H9NOCl
A) B
B) C
C) A

-C8H9NOCl
A) B
B) C
C) A

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
6
Match the following molecular formulae to the mass spectra A-C:

-C10H21NO
A) B
B) C
C) A

-C10H21NO
A) B
B) C
C) A

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following bonds has the strongest IR absorption?

A) A.
B) B.
C) C.
D) D.

A) A.
B) B.
C) C.
D) D.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
8
Match the following aliphatic molecules to their infrared carbonyl stretching frequency:

-1815 cm-1
A) A
B) C
C) D
D) B

-1815 cm-1
A) A
B) C
C) D
D) B

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
9
Match the following aliphatic molecules to their infrared carbonyl stretching frequency:

-1740 cm-1
A) A
B) C
C) D
D) B

-1740 cm-1
A) A
B) C
C) D
D) B

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
10
Match the following aliphatic molecules to their infrared carbonyl stretching frequency:

-1715 cm-1
A) A
B) C
C) D
D) B

-1715 cm-1
A) A
B) C
C) D
D) B

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
11
Match the following aliphatic molecules to their infrared carbonyl stretching frequency:

-1650 cm-1
A) A
B) C
C) D
D) B

-1650 cm-1
A) A
B) C
C) D
D) B

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
12
From the following molecules, pick out those which have no infrared absorption for a symmetrical stretch involving their corresponding double/triple bond(s). Please select all that apply.
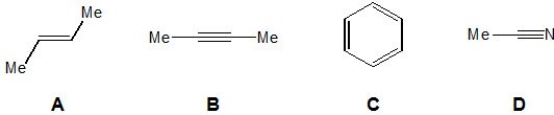
A) A.
B) B.
C) C.
D) D.
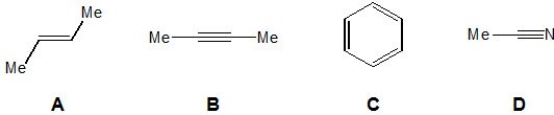
A) A.
B) B.
C) C.
D) D.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
13
From the following molecules, pick out those which have four proton signals in their 1H NMR spectrum. Please select all that apply.
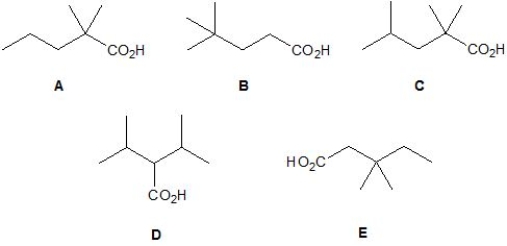
A) A.
B) B.
C) C.
D) D.
E) E.

A) A.
B) B.
C) C.
D) D.
E) E.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
14
From the following molecules, pick out those which do not have four proton signals in their 1H NMR spectrum. Please select all that apply.
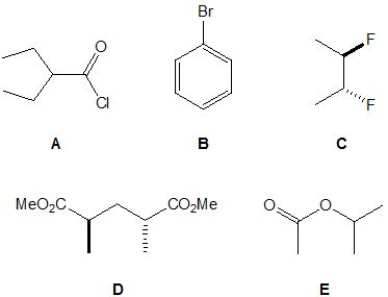
A) A.
B) B.
C) C.
D) D.
E) E.

A) A.
B) B.
C) C.
D) D.
E) E.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
15
How many magnetically non-equivalent carbon atoms are there in toluene?
A) 4.
B) 5.
C) 6.
D) 7.
A) 4.
B) 5.
C) 6.
D) 7.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
16
How many magnetically non-equivalent carbon atoms are there in the following molecule?
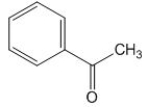
A) 5.
B) 8.
C) 4.
D) 6.

A) 5.
B) 8.
C) 4.
D) 6.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
17
How many magnetically non-equivalent carbon atoms are there in the following molecule?
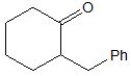
A) 7.
B) 8.
C) 10.
D) 11.
E) 13.

A) 7.
B) 8.
C) 10.
D) 11.
E) 13.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
18
From the following molecules, pick out those which have six carbon signals in their 13C NMR spectrum. Please select all that apply.
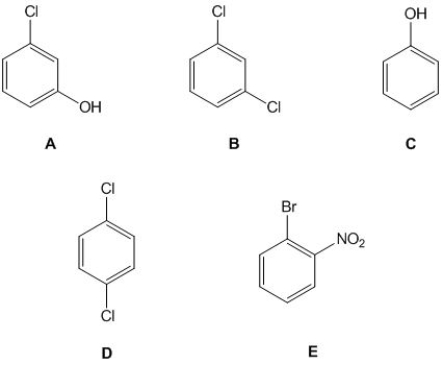
A) A.
B) B.
C) C.
D) D.
E) E.

A) A.
B) B.
C) C.
D) D.
E) E.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
19
For an unknown compound, its proton and carbon NMR spectra showed three proton and four carbon signals, respectively. Pick out this unknown compound from the following molecules.
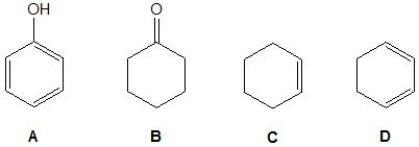
A) A.
B) B.
C) C.
D) D.

A) A.
B) B.
C) C.
D) D.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
20
The following molecules were examined by 1H NMR spectroscopy. Rank them in order of decreasing chemical shift for their methyl group (where 1 indicates the largest ppm and 3 indicates the smallest ppm):
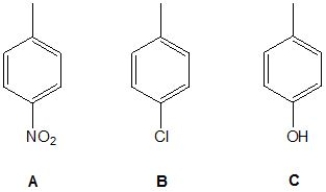
A) Molecule A
B) Molecule B
C) Molecule C

A) Molecule A
B) Molecule B
C) Molecule C

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
21
Which one of the following 1H NMR signals is a quintet?
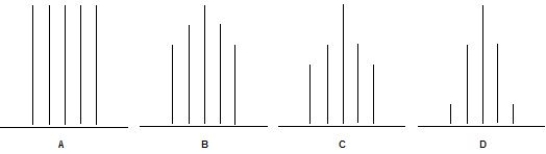
A) A.
B) B.
C) C.
D) D.

A) A.
B) B.
C) C.
D) D.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
22
By 1H NMR spectroscopy, what is the expected signal intensity for the eight lines of an octet?
A) 1:1:1:1:1:1:1:1.
B) 1:2:3:4:4:3:2:1.
C) 1:3:6:12:12:6:3:1.
D) 1:5:10:15:15:10:5:1.
E) 1:7:21:35:35:21:7:1.
A) 1:1:1:1:1:1:1:1.
B) 1:2:3:4:4:3:2:1.
C) 1:3:6:12:12:6:3:1.
D) 1:5:10:15:15:10:5:1.
E) 1:7:21:35:35:21:7:1.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
23
How many degrees of unsaturation are there in a compound with the molecular formula C6H10NOCl?
A) 1.
B) 2.
C) 3.
D) 4.
E) 5.
A) 1.
B) 2.
C) 3.
D) 4.
E) 5.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
24
An unknown compound is analysed using NMR spectroscopy, generating the NMR spectra shown below. What is the structure of the unknown compound?
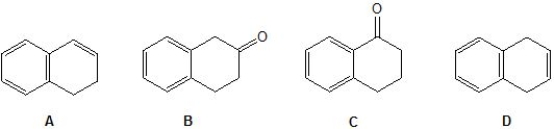
1H NMR spectrum of unknown compound
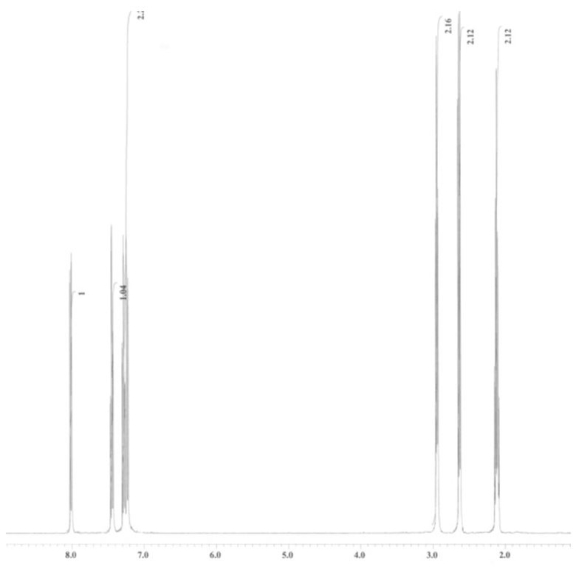
13C NMR spectrum of unknown compound
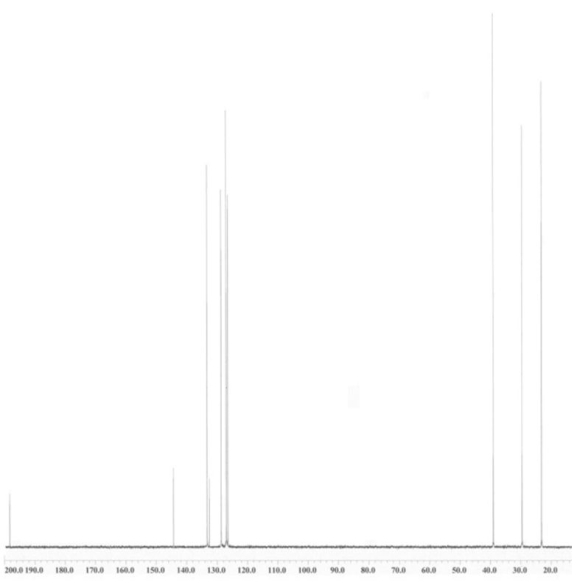
1H NMR expansion 1
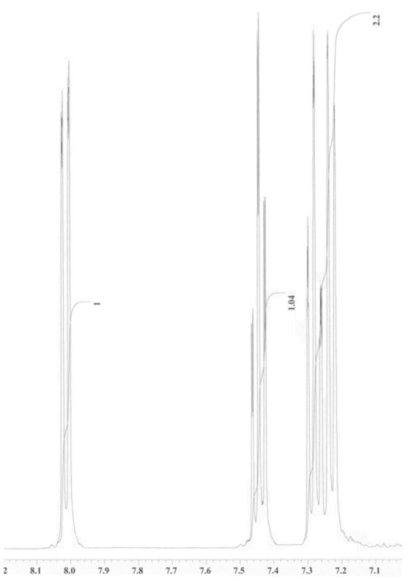
1H NMR expansion 2
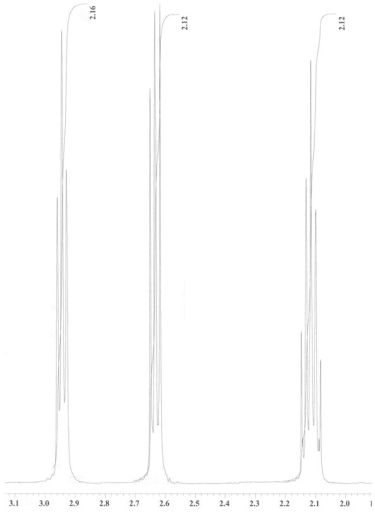
A) A.
B) B.
C) C.
D) D.

1H NMR spectrum of unknown compound

13C NMR spectrum of unknown compound

1H NMR expansion 1

1H NMR expansion 2

A) A.
B) B.
C) C.
D) D.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
25
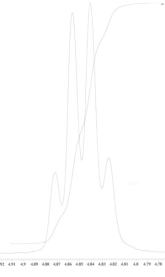
An unknown compound is analysed using NMR spectroscopy, generating the NMR spectra shown below. What is the structure of the unknown compound?
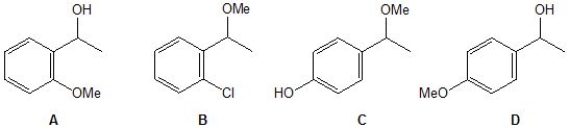
1H NMR spectrum of unknown compound
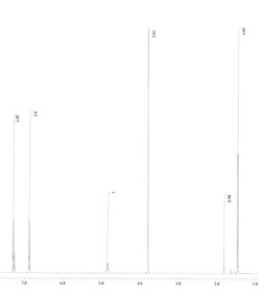
13C NMR spectrum of unknown compound
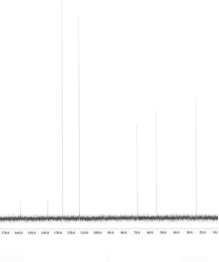
1H NMR expansion 1
1H NMR expansion 2
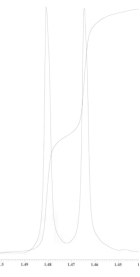
1H NMR expansion 3
A) A.
B) B.
C) C.
D) D.

1H NMR spectrum of unknown compound

13C NMR spectrum of unknown compound

1H NMR expansion 1

1H NMR expansion 2

1H NMR expansion 3

A) A.
B) B.
C) C.
D) D.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
26
Select all of the following statements on expected IR frequencies that are true. Please select all that apply.
A) Acid chlorides will appear between 1820 - 1650 cm-1
B) Esters will appear between 3600 - 2500 cm-1
C) Phenols will appear between 3600 - 2500 cm-1
D) Aromatic C-H will appear between 3000 - 2850 cm-1
A) Acid chlorides will appear between 1820 - 1650 cm-1
B) Esters will appear between 3600 - 2500 cm-1
C) Phenols will appear between 3600 - 2500 cm-1
D) Aromatic C-H will appear between 3000 - 2850 cm-1

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
27
Select the following analytical techniques that would allow a pair of enantiomers of a molecule to be distinguished. Please select all that apply.
A) Chiral liquid chromatography
B) Mass Spectrometry
C) 1H-NMR
D) Polarimetry
A) Chiral liquid chromatography
B) Mass Spectrometry
C) 1H-NMR
D) Polarimetry

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
28
Select the molecule from the following structure that will have 6 carbon peaks, 4 hydrogen peaks in its NMR spectra.
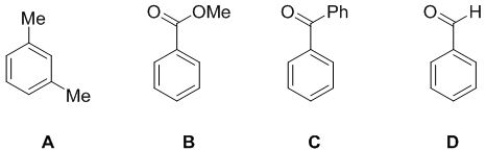
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
29
Based on the information given, select the molecule to which it fits best.
7 carbon and 5 hydrogen peaks in its NMR spectra. MS 183.95 (100 %), 185.95 (100 %). IR frequency at 1740 cm-1
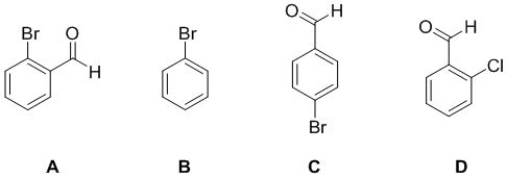
A) A
B) B
C) C
D) D
7 carbon and 5 hydrogen peaks in its NMR spectra. MS 183.95 (100 %), 185.95 (100 %). IR frequency at 1740 cm-1

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
30
Select the two major factors which influence chemical shifts from the list below. Please select all that apply.
A) Electron density
B) Anisotropic effects
C) Symmetry effects
D) Number of adjacent atoms
A) Electron density
B) Anisotropic effects
C) Symmetry effects
D) Number of adjacent atoms

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck


