Deck 8: Gases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/38
Play
Full screen (f)
Deck 8: Gases
1
What is the pressure exerted by a column of mercury 43 cm high, given that the density of mercury is 13.6 g cm-3? Give your answer in base SI units.
A) 5736.89 g cm-2 m s-2.
B) 57.37 g cm-3 m2 s-2.
C) 57368.88 kg m-1 s-2.
D) 5736888 g cm-1 s-2.
A) 5736.89 g cm-2 m s-2.
B) 57.37 g cm-3 m2 s-2.
C) 57368.88 kg m-1 s-2.
D) 5736888 g cm-1 s-2.
C
2
Which of the following units are commonly used for pressure? Select all that apply.
A) Pa.
B) bar.
C) kg m s-2.
D) N m-2.
A) Pa.
B) bar.
C) kg m s-2.
D) N m-2.
A, B, D
3
At constant temperature, the volume of a fixed amount of gas ______________ with increasing pressure.
decreases
4
As the temperature of a fixed amount of gas, increases at constant pressure, its volume decreases.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
5
Avogadro's law states that at constant pressure and temperature, a different number of molecules will be present in equal volumes of oxygen and carbon dioxide.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
6
Calculate the pressure (in Pa) required to compress 0.25 dm3 of a gas at 1 atm to a volume of 0.10 dm3 at the same temperature?
A) 2.5
B) 2.5 × 105.
C) 1.0
D) 1.01 × 105.
A) 2.5
B) 2.5 × 105.
C) 1.0
D) 1.01 × 105.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
7
What would be the volume (in dm3 ) of N2 at 293 K and 720 Torr if it occupied 12.5 dm3 at 329 K and 0.947 atm?
A) 14.04
B) 12.5
C) 11.13
D) 11.84
A) 14.04
B) 12.5
C) 11.13
D) 11.84

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
8
A balloon is filled at 1 atm with gas that occupies 2.3 dm3 in a laboratory at 19 °C. The balloon is then taken from this laboratory to a tropical greenhouse at 1 atm where the temperature is 38 °C. What is the resulting volume (dm3) of the balloon?
A) 4.6
B) 2.3
C) 2.16
D) 2.45
A) 4.6
B) 2.3
C) 2.16
D) 2.45

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
9
Combining Boyle's law, Charles's law and Avogadro's law gives the _____ gas equation.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
10
The gas constant R can be expressed in different units. Select all that apply.
A) N m K-1 mol-1.
B) dm3 atm mol-1 K-1.
C) J K-1 mol-1.
D) dm3 Torr K-1 mol-1.
A) N m K-1 mol-1.
B) dm3 atm mol-1 K-1.
C) J K-1 mol-1.
D) dm3 Torr K-1 mol-1.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
11
A 28.0 dm3 gas cylinder at 5.2 atm and 23 °C contains only carbon monoxide gas. What is the mass (in kg) of carbon monoxide in the tank?
A) 168.
B) 0.168
C) 0.021
D) 1.656 × 10-6.
A) 168.
B) 0.168
C) 0.021
D) 1.656 × 10-6.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
12
A balloon is filled with 39.3 g of helium at 29 °C and 2.75 atm. What is the volume of the balloon in dm3?
A) 88.52
B) 8.852 × 10-2.
C) 8.97 × 106.
D) 8.50
A) 88.52
B) 8.852 × 10-2.
C) 8.97 × 106.
D) 8.50

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
13
A 5.2 dm3 gas cylinder containing 822 g of neon is heated until the pressure reaches 232 atm. What is the temperature in °C of the gas?
A) - 273.15
B) 87.80
C) 360.95
D) -255.26
A) - 273.15
B) 87.80
C) 360.95
D) -255.26

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
14
Equal volumes of carbon dioxide and a second gas weigh 32.00 g and 14.67 g respectively under the same experimental conditions. Which of the following is the unknown gas?
A) He
B) O2
C) Ne
D) Ar
A) He
B) O2
C) Ne
D) Ar

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
15
A small bottle of nitrogen gas, volume 6.5 dm3 is stored at 22 °C and a pressure of 63 bar. Calculate the pressure (in bar) inside the bottle when the temperature increases to 28 °C; assume the gas behaves as an ideal gas.
A) 49.5
B) Not enough information to calculate the new pressure.
C) 7.08 × 10-4
D) 64.28
A) 49.5
B) Not enough information to calculate the new pressure.
C) 7.08 × 10-4
D) 64.28

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
16
A sealed reaction vessel volume is 0.5 dm3 contains argon gas at 1 atm and 21 °C. Assuming that argon behaves ideally under these conditions calculate the mass of Ar in the reaction vessel.
A) 0.11 g.
B) 0.0005 g.
C) 0.83 g.
D) 8.17 × 10-6 g.
A) 0.11 g.
B) 0.0005 g.
C) 0.83 g.
D) 8.17 × 10-6 g.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
17
A reaction vessel is fitted with a syringe. It contains gas at 1 atm and 18 °C. Calculate the pressure if the syringe is moved so that it is a quarter of its original volume. Assume temperature to be constant.
A) 1 atm.
B) 0.25 atm.
C) 101 325 Pa.
D) 4 atm
A) 1 atm.
B) 0.25 atm.
C) 101 325 Pa.
D) 4 atm

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
18
The IUPAC definition of standard ambient temperature and pressure, SATP, is 298.15 K and 1 atm.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
19
Calculate the molar volume of one mole of an ideal gas under conditions of standard ambient pressure and temperature.
A) 0.0248 m3 .
B) 0.0245 m3.
C) 0.0227 m3 .
D) 0.0224 m3.
A) 0.0248 m3 .
B) 0.0245 m3.
C) 0.0227 m3 .
D) 0.0224 m3.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
20
A gas-mixing cylinder is filled with a mixture of He, Ne and Ar, in a 1:1:2 ratio. The total pressure in the gas-mixing cylinder is 3.29 × 105 Pa. Calculate the partial pressure of helium in the mixture.
A) 164500 Pa.
B) 75000 Pa.
C) 82250 Pa.
D) 25000 Pa.
A) 164500 Pa.
B) 75000 Pa.
C) 82250 Pa.
D) 25000 Pa.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
21
The kinetic molecular theory describes gas behaviour in terms of the movement of molecules. In developing the model, which assumptions were made? Select all that apply.
A) The motion of the gas molecules is constant and random.
B) Gas molecules have negligible size compared to the vessel in which the gas is contained.
C) Any collisions between the molecules and the walls of the container will increase the energy of the molecules.
D) The molecules do not interact with each other.
A) The motion of the gas molecules is constant and random.
B) Gas molecules have negligible size compared to the vessel in which the gas is contained.
C) Any collisions between the molecules and the walls of the container will increase the energy of the molecules.
D) The molecules do not interact with each other.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
22
Calculate the root mean square speed of argon gas at 15 °C. Give your answer in m s-1.
A) 96.
B) 424.
C) 13.
D) 3.
A) 96.
B) 424.
C) 13.
D) 3.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
23
Calculate the root mean square speed of ammonia gas at 17 °C. Give your answer in m s-1.
A) 158.
B) 21.
C) 5.
D) 652.
A) 158.
B) 21.
C) 5.
D) 652.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
24
A 0.25 dm3 sealed container is filled with 3 mol of He, with a root mean square molecular speed, c = 1250 m s-1. Find the pressure exerted by the gas (in k Pa).
A) 25.02
B) 0.25 × 105.
C) 1.51 × 1028.
D) 1.51 × 1025.
A) 25.02
B) 0.25 × 105.
C) 1.51 × 1028.
D) 1.51 × 1025.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
25
The molecular speed distribution of neon will change with temperature. Select all that apply.
A) At higher temperatures, the average speed will be greater than at lower temperatures.
B) At higher temperatures, the spread of speeds will be greater than at lower temperatures.
C) At lower temperatures, the distribution of speeds will be greater than at higher temperatures.
D) At lower temperatures, the average speed will be greater than at higher temperatures.
A) At higher temperatures, the average speed will be greater than at lower temperatures.
B) At higher temperatures, the spread of speeds will be greater than at lower temperatures.
C) At lower temperatures, the distribution of speeds will be greater than at higher temperatures.
D) At lower temperatures, the average speed will be greater than at higher temperatures.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
26
1 mol of Xe gas at 345 K will have a greater distribution of molecular speeds and higher average speed than 1 mol of He at the same temperature.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
27
Rank the following gases, in order of rate of effusion (from highest to lowest rate) through a porous film under the same experimental conditions.
A) O3
B) O2
C) CO2
D) CO
A) O3
B) O2
C) CO2
D) CO

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
28
35 mol of nitrogen gas passes through a membrane in 30 minutes. How long will it take for the same amount of carbon dioxide to pass through the same membrane? Give your answer to the nearest whole minute.
A) 38 min.
B) 24 min.
C) 47 min.
D) 19 min.
A) 38 min.
B) 24 min.
C) 47 min.
D) 19 min.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
29
Calculate the relative rates of effusion of helium, neon, argon, and xenon through a gas cylinder regulator.
A) 1:2.25:3.16:5.73
B) 1:0.45:0.32:0.17
C) 1:0.20:0.10:0.03
D) 1:0.45:0.71:0.55
A) 1:2.25:3.16:5.73
B) 1:0.45:0.32:0.17
C) 1:0.20:0.10:0.03
D) 1:0.45:0.71:0.55

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
30
Match the characteristic property of molecules in a gas with its description.
-collision cross section, σ
A) target area of a molecule
B) number of collisions per second
C) average distance travelled by a molecule between collisions
D) product of collision frequency and mean free path
-collision cross section, σ
A) target area of a molecule
B) number of collisions per second
C) average distance travelled by a molecule between collisions
D) product of collision frequency and mean free path

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
31
Match the characteristic property of molecules in a gas with its description.
-collision frequency, Z
A) target area of a molecule
B) number of collisions per second
C) average distance travelled by a molecule between collisions
D) product of collision frequency and mean free path
-collision frequency, Z
A) target area of a molecule
B) number of collisions per second
C) average distance travelled by a molecule between collisions
D) product of collision frequency and mean free path

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
32
Match the characteristic property of molecules in a gas with its description.
-mean free path, λ
A) target area of a molecule
B) number of collisions per second
C) average distance travelled by a molecule between collisions
D) product of collision frequency and mean free path
-mean free path, λ
A) target area of a molecule
B) number of collisions per second
C) average distance travelled by a molecule between collisions
D) product of collision frequency and mean free path

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
33
Match the characteristic property of molecules in a gas with its description.
-mean speed,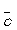
A) target area of a molecule
B) number of collisions per second
C) average distance travelled by a molecule between collisions
D) product of collision frequency and mean free path
-mean speed,
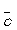
A) target area of a molecule
B) number of collisions per second
C) average distance travelled by a molecule between collisions
D) product of collision frequency and mean free path

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
34
How many collisions will O2 undergo in 1 s at SATP? (For O2 σ = 0.40 nm2, 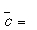 444 m s-1 at 298 K.)
444 m s-1 at 298 K.)
A) 6.1 × 109.
B) 6.1 × 1027.
C) 6.1 × 1018.
D) 6.1 × 104.
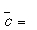 444 m s-1 at 298 K.)
444 m s-1 at 298 K.)A) 6.1 × 109.
B) 6.1 × 1027.
C) 6.1 × 1018.
D) 6.1 × 104.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
35
Calculate the pressure (in Pa) at which O2 has a mean free path of 0.5 cm. (For O2, σ = 0.40 nm2, 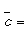 444 m s-1 at 298 K).
444 m s-1 at 298 K).
A) 1.46 × 10-17.
B) 1.46 × 10-8.
C) 1.46
D) 1.46 × 10-2.
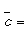 444 m s-1 at 298 K).
444 m s-1 at 298 K).A) 1.46 × 10-17.
B) 1.46 × 10-8.
C) 1.46
D) 1.46 × 10-2.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
36
The ideal gas equation is an example of a limiting law. It describes well the behaviour of gases as gas pressure tends to ____.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
37
The van der Waals equation for gases is 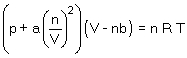 The van der Waals constants a and b attempt to correct for the behaviour of real gases relative to ideal behaviour. The constants correct for which of the following: select all that apply.
The van der Waals constants a and b attempt to correct for the behaviour of real gases relative to ideal behaviour. The constants correct for which of the following: select all that apply.
A) The reduced pressure of real gases with respect to ideal gases.
B) The increased pressure of real gases with respect to ideal gases.
C) The reduced volume available for real gas molecules to move in.
D) The increased volume available for real gas molecules to move in.
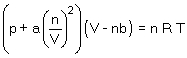 The van der Waals constants a and b attempt to correct for the behaviour of real gases relative to ideal behaviour. The constants correct for which of the following: select all that apply.
The van der Waals constants a and b attempt to correct for the behaviour of real gases relative to ideal behaviour. The constants correct for which of the following: select all that apply.A) The reduced pressure of real gases with respect to ideal gases.
B) The increased pressure of real gases with respect to ideal gases.
C) The reduced volume available for real gas molecules to move in.
D) The increased volume available for real gas molecules to move in.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
38
A reaction vessel (volume 0.5 dm3) is filled with 3 mol of hydrogen gas at 25 °C. Calculate the pressure, (in Pa) it exerts using the van der Waals equation. For hydrogen, the van der Waals constants are a = 0.025 Pa m6 mol-2 and b = 2.7 × 10-5 m3 mol-1.
A) 1.68 × 107.
B) 1.49 × 107.
C) 5.88 × 105.
D) - 9 × 105.
A) 1.68 × 107.
B) 1.49 × 107.
C) 5.88 × 105.
D) - 9 × 105.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck


