Deck 5: Spectroscopy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/41
Play
Full screen (f)
Deck 5: Spectroscopy
1
An analyst is tasked with determining the zinc content in a bullet casing. What category of spectroscopy is best suited to the task?
A) atomic
B) molecular
C) interactive
D) proactive
E) none of the above
A) atomic
B) molecular
C) interactive
D) proactive
E) none of the above
A
2
What is the best category of spectroscopy to use to study the structure of a previously unknown drug?
A) atomic
B) molecular
C) interactive
D) proactive
E) none of the above
A) atomic
B) molecular
C) interactive
D) proactive
E) none of the above
B
3
The lower the energy of electromagnetic energy, the
A) shorter the wavelength
B) greater the energy of the photon
C) longer the wavelength
D) higher the frequency
E) none of the above
A) shorter the wavelength
B) greater the energy of the photon
C) longer the wavelength
D) higher the frequency
E) none of the above
C
4
The higher the energy of electromagnetic energy, the
A) shorter the wavelength
B) smaller the energy of the photon
C) longer the wavelength
D) higher the frequency
E) none of the above
A) shorter the wavelength
B) smaller the energy of the photon
C) longer the wavelength
D) higher the frequency
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
5
Given the following information:
Planck's constant = 6.626x10-34 J s
1 nm = 10-9m
Speed of light = 3x108m/s
Calculate the frequency (s-1) of energy visible range with a wavelength of 330 nm
A) 9.1x10-7
B) 1.0x109
C) 9.1x1014
D) 99.
E) none of the above
Planck's constant = 6.626x10-34 J s
1 nm = 10-9m
Speed of light = 3x108m/s
Calculate the frequency (s-1) of energy visible range with a wavelength of 330 nm
A) 9.1x10-7
B) 1.0x109
C) 9.1x1014
D) 99.
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
6
Given the following information:
Planck's constant = 6.626x10-34 J s
1 nm = 10-9m
Speed of light = 3x108m/s
100 cm per meter
Calculate the wavelength (cm) of energy in the IR range with a frequency 8.4x1012s-1
A) 3.6x10-3
B) 280
C) 2.8x104
D) 3.6x10-3
E) none of the above
Planck's constant = 6.626x10-34 J s
1 nm = 10-9m
Speed of light = 3x108m/s
100 cm per meter
Calculate the wavelength (cm) of energy in the IR range with a frequency 8.4x1012s-1
A) 3.6x10-3
B) 280
C) 2.8x104
D) 3.6x10-3
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
7
Given the following information:
Planck's constant = 6.626x10-34 J s
1 nm = 10-9m
Speed of light = 3x108m/s
100 cm per meter
Calculate the wavenumber (cm-1) of energy in the IR range with a frequency of 1.54x1012s-1
A) 3.6x10-3
B) 51
C) 1.9x10-2
D) 1.9x10-4
E) none of the above
Planck's constant = 6.626x10-34 J s
1 nm = 10-9m
Speed of light = 3x108m/s
100 cm per meter
Calculate the wavenumber (cm-1) of energy in the IR range with a frequency of 1.54x1012s-1
A) 3.6x10-3
B) 51
C) 1.9x10-2
D) 1.9x10-4
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
8
Given the following information:
Planck's constant = 6.626x10-34 J s
1 nm = 10-9m
Speed of light = 3x108m/s
100 cm per meter
Calculate the energy of a photon of x-ray energy (J) with a frequency of 3.9x1019s-1
A) 1.7x10-53
B) 22
C) 4.1x1011
D) 4.1x10-14
E) none of the above
Planck's constant = 6.626x10-34 J s
1 nm = 10-9m
Speed of light = 3x108m/s
100 cm per meter
Calculate the energy of a photon of x-ray energy (J) with a frequency of 3.9x1019s-1
A) 1.7x10-53
B) 22
C) 4.1x1011
D) 4.1x10-14
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
9
Given the following information:
Planck's constant = 6.626x10-34 J s
1 nm = 10-9m
Speed of light = 3x108m/s
100 cm per meter
Calculate the energy of a photon (J) of radio energy (used in NMR) with a wavelength of 0.95m
A) 6.3x10-34
B) 2.1x10-25
C) 75
D) 4.8x1024
E) none of the above
Planck's constant = 6.626x10-34 J s
1 nm = 10-9m
Speed of light = 3x108m/s
100 cm per meter
Calculate the energy of a photon (J) of radio energy (used in NMR) with a wavelength of 0.95m
A) 6.3x10-34
B) 2.1x10-25
C) 75
D) 4.8x1024
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
10
What does it mean when we say that absorbance is quantized?
A) Within a system, energy increases or decreases in a continuous smooth curve
B) There are discrete energy levels within a given system
C) The energy is quantitated and well understood
D) Energy can only be reflected if it is below a specific quantity
E) none of the above
A) Within a system, energy increases or decreases in a continuous smooth curve
B) There are discrete energy levels within a given system
C) The energy is quantitated and well understood
D) Energy can only be reflected if it is below a specific quantity
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
11
Suppose a molecule in a solution absorbs a photon and an electron is promoted to an excited state, M*. If the electron immediately falls back to the ground state, what else must happen?
A) the molecule starts vibrating
B) photon emission
C) the molecule starts rotating
D) other electrons must cascade into lower energy levels
E) none of the above
A) the molecule starts vibrating
B) photon emission
C) the molecule starts rotating
D) other electrons must cascade into lower energy levels
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
12
Why does the process M* → M occur in spectroscopy?
A) the ground state is unstable
B) photons cannot stay attached to M
C) temperature is always above absolute zero
D) the excited state is unstable
E) none of the above
A) the ground state is unstable
B) photons cannot stay attached to M
C) temperature is always above absolute zero
D) the excited state is unstable
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
13
What are two methods by which a molecule or atom in an excited state can release energy and return to the ground state?
A) photon emission and collision
B) photon absorbance and collision
C) collision and evaporation
D) absorbance and emission
E) none of the above
A) photon emission and collision
B) photon absorbance and collision
C) collision and evaporation
D) absorbance and emission
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
14
What is Beer's Law?
A) The relationship that describes absorbance in terms of quantity of an analyte
B) Another way to express the second law of thermodynamics
C) The relationship between frequency and wavelength
D) The relationship between photons and wavelength
E) none of the above
A) The relationship that describes absorbance in terms of quantity of an analyte
B) Another way to express the second law of thermodynamics
C) The relationship between frequency and wavelength
D) The relationship between photons and wavelength
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
15
The following is a basic diagram of a spectrometer.
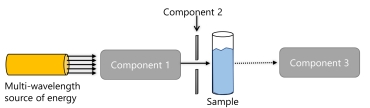 What is component 1?
What is component 1?
A) a slit
B) an interferometer
C) a monochromator
D) a detector
E) none of the above
 What is component 1?
What is component 1?A) a slit
B) an interferometer
C) a monochromator
D) a detector
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
16
The following is a basic diagram of a spectrometer. 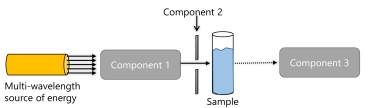 What is component 3?
What is component 3?
A) a slit
B) an interferometer
C) a monochromator
D) a detector
E) none of the above
 What is component 3?
What is component 3?A) a slit
B) an interferometer
C) a monochromator
D) a detector
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
17
The following is a basic diagram of a spectrometer.
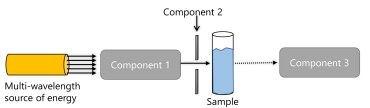 What is component 2?
What is component 2?
A) a slit
B) an interferometer
C) a monochromator
D) a detector
E) none of the above
 What is component 2?
What is component 2?A) a slit
B) an interferometer
C) a monochromator
D) a detector
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
18
The following is a basic diagram of a spectrometer.
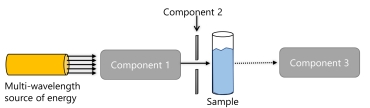 What happens to the energy in the sample?
What happens to the energy in the sample?
A) energy is absorbed and less than the source intensity is transmitted to the detector.
B) energy is absorbed and more than the source intensity is transmitted to the detector.
C) interference occurs and the interferogram is transmitted to the detector
D) energy is absorbed and additional photons are emitted, resulting in a greater intensity being sent to the detector
E) none of the above
 What happens to the energy in the sample?
What happens to the energy in the sample?A) energy is absorbed and less than the source intensity is transmitted to the detector.
B) energy is absorbed and more than the source intensity is transmitted to the detector.
C) interference occurs and the interferogram is transmitted to the detector
D) energy is absorbed and additional photons are emitted, resulting in a greater intensity being sent to the detector
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
19
Which monochromator uses mathematical operators to separate wavelengths?
A) prism
B) interferometer
C) grating
D) slit
E) none of the above
A) prism
B) interferometer
C) grating
D) slit
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
20
What is the tradeoff associated with slit width in a spectrometer?
A) a wide slit decreases signal intensity but increases resolution
B) a narrow slit reduces stray light but decreases signal intensity
C) a narrow slit increases intensity but decreases resolution
D) a wide slit increases intensity but decreases spectral efficiency
E) none of the above
A) a wide slit decreases signal intensity but increases resolution
B) a narrow slit reduces stray light but decreases signal intensity
C) a narrow slit increases intensity but decreases resolution
D) a wide slit increases intensity but decreases spectral efficiency
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
21
What type of spectrometer uses a separate beam path for a reference sample?
A) double beam
B) single beam repeater
C) prism dispersion
D) grating dispersion
E) none of the above
A) double beam
B) single beam repeater
C) prism dispersion
D) grating dispersion
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
22
What is the spectral bandwidth?
A) the resolution of the detector
B) the ability of a prism to resolve colors
C) the detection limit of the detector
D) the width of the radiation band emanating from the source or monochromator
E) none of the above
A) the resolution of the detector
B) the ability of a prism to resolve colors
C) the detection limit of the detector
D) the width of the radiation band emanating from the source or monochromator
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
23
Ideally, the spectral bandwidth should be ________ compared to what the target analyte will absorb
A) smaller
B) the same
C) larger
D) less intense
E) none of the above
A) smaller
B) the same
C) larger
D) less intense
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
24
A UV spectrometer is capable of producing two distinct peaks for wavelengths 253 and 255. What is the resolution of this instrument in this range?
A) 127
B) 2
C) 0.01
D) 100
E) none of the above
A) 127
B) 2
C) 0.01
D) 100
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
25
What type of atoms or molecules generate a UV spectrum?
A) those with single bonds
B) those with k,l, or m electron shells
C) those with π electrons
D) those with free radicals
E) none of the above
A) those with single bonds
B) those with k,l, or m electron shells
C) those with π electrons
D) those with free radicals
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
26
When a UV photon is absorbed by a molecule, what happens?
A) an electron can be kicked out of an inner shell
B) an electron can be promoted within the molecular orbital structure
C) a molecule can be promoted to a higher energy vibrational state
D) a molecule can be promoted to an unstable rotational state
E) none of the above
A) an electron can be kicked out of an inner shell
B) an electron can be promoted within the molecular orbital structure
C) a molecule can be promoted to a higher energy vibrational state
D) a molecule can be promoted to an unstable rotational state
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
27
When an IR photon is absorbed by a molecule, what happens?
A) an electron can be kicked out of an inner shell
B) an electron can be promoted within the molecular orbital structure
C) a molecule can be promoted to a higher energy vibrational state
D) a molecule can be promoted to an unstable rotational state
E) none of the above
A) an electron can be kicked out of an inner shell
B) an electron can be promoted within the molecular orbital structure
C) a molecule can be promoted to a higher energy vibrational state
D) a molecule can be promoted to an unstable rotational state
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
28
IR spectroscopy reveals information about all of the following except
A) types of bonds
B) functional groups present
C) vibrational characteristics of bonds
D) molecular weight
E) none of the above
A) types of bonds
B) functional groups present
C) vibrational characteristics of bonds
D) molecular weight
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
29
Interferometry, as applied in FTIR, uses what type of monochromator?
A) Michelson interferometer
B) prism
C) grating
D) selected filters for the source
E) none of the above
A) Michelson interferometer
B) prism
C) grating
D) selected filters for the source
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
30
How does an interferometry in FTIR generate interference patterns?
A) high intensity lasers
B) offset gratings
C) two light paths, one with a moving mirror
D) mathematical trickery
E) none of the above
A) high intensity lasers
B) offset gratings
C) two light paths, one with a moving mirror
D) mathematical trickery
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
31
The Fourier transform in FTIR is used to
A) convert wavelengths to frequencies
B) convert information in the time domain to the frequency domain
C) generate the interferogram
D) convert photons to electrons
E) none of the above
A) convert wavelengths to frequencies
B) convert information in the time domain to the frequency domain
C) generate the interferogram
D) convert photons to electrons
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
32
What type of interaction does Raman spectroscopy detect?
A) scattered
B) reflected
C) transmitted
D) absorbed
E) none of the above
A) scattered
B) reflected
C) transmitted
D) absorbed
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
33
Why are lasers used as the source in Raman spectroscopy?
A) they use a tight beam
B) scattering interactions are strong so a strong source is required
C) they have become relatively inexpensive
D) scattering interactions are very weak
E) none of the above
A) they use a tight beam
B) scattering interactions are strong so a strong source is required
C) they have become relatively inexpensive
D) scattering interactions are very weak
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
34
What range of electromagnetic radiation is used in NMR spectroscopy?
A) X-ray
B) UV-VIS
C) IR
D) radiofrequency
E) none of the above
A) X-ray
B) UV-VIS
C) IR
D) radiofrequency
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
35
What interaction is targeted in NMR spectroscopy?
A) nuclear motion in a magnetic field
B) promotion of electrons within molecular orbitals
C) promotion of electrons within atomic orbitals
D) ejection of electrons from inner shell atomic orbitals
E) none of the above
A) nuclear motion in a magnetic field
B) promotion of electrons within molecular orbitals
C) promotion of electrons within atomic orbitals
D) ejection of electrons from inner shell atomic orbitals
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
36
How is an electrical current generated and detected in NMR?
A) photons are converted to electrons by the detector
B) electrons fall back into the ground state and release a photon
C) as the spin axis changes, the charged nucleus induces a current in a coil
D) radiofrequency causes electrons to oscillate in an antenna
E) none of the above
A) photons are converted to electrons by the detector
B) electrons fall back into the ground state and release a photon
C) as the spin axis changes, the charged nucleus induces a current in a coil
D) radiofrequency causes electrons to oscillate in an antenna
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
37
When an electronegative atom pulls electrons away from a neighboring atom, this results in
A) bond breaking
B) free radical formation
C) conjugation
D) deshielding
E) none of the above
A) bond breaking
B) free radical formation
C) conjugation
D) deshielding
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
38
H-NMR spectroscopy reveals all of the following except
A) number of hydrogens in a molecule
B) how they are arranged
C) molecular structure
D) vibrational energy levels
E) none of the above
A) number of hydrogens in a molecule
B) how they are arranged
C) molecular structure
D) vibrational energy levels
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
39
What does a scanning electron microscope use to "illuminate" a sample surface?
A) an electron beam
B) an X-ray beam
C) a laser
D) multiple lasers
E) none of the above
A) an electron beam
B) an X-ray beam
C) a laser
D) multiple lasers
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
40
Why are X-ray spectroscopy detectors commonly associated with SEM instruments?
A) both use X-rays as a source
B) both use lasers on the sample surface
C) the interaction of electrons with a surface can generate a X-ray spectrum
D) they both rely on high magnification
E) none of the above
A) both use X-rays as a source
B) both use lasers on the sample surface
C) the interaction of electrons with a surface can generate a X-ray spectrum
D) they both rely on high magnification
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
41
Why do some elements generate more than one peak in X-ray spectroscopy?
A) when an electron is ejected from the atom, one falls in to fill the gap, which generates another gap and and another electron falling
B) electrons can be ejected and rebasorbed many times
C) bonds break and change the electron configurations of some atoms
D) electron bombardment can temporarily change a nucleus to another element
E) none of the above
A) when an electron is ejected from the atom, one falls in to fill the gap, which generates another gap and and another electron falling
B) electrons can be ejected and rebasorbed many times
C) bonds break and change the electron configurations of some atoms
D) electron bombardment can temporarily change a nucleus to another element
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck


