Deck 9: The Basics of Chemical Bonding
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/167
Play
Full screen (f)
Deck 9: The Basics of Chemical Bonding
1
Which of the following statements is true?
A)Bond formation tends to increase the potential energy of the atoms involved.
B)Compounds with negative heats of formation tend to be unstable with respect to decomposition into their elements.
C)When H°f is negative, the reaction produces a stable compound.
D)An endothermic reaction produces a fairly stable compound.
E)Compounds with positive heats of formation tend to be stable with respect to decomposition into their elements.
A)Bond formation tends to increase the potential energy of the atoms involved.
B)Compounds with negative heats of formation tend to be unstable with respect to decomposition into their elements.
C)When H°f is negative, the reaction produces a stable compound.
D)An endothermic reaction produces a fairly stable compound.
E)Compounds with positive heats of formation tend to be stable with respect to decomposition into their elements.
When H°f is negative, the reaction produces a stable compound.
2
Based on the H°f data given, which compound is the most stable?
A)N2O4(g), +9.7 kJ/mol
B)H2S(g), -20.6 kJ/mol
C)N2H4(g), +94.5 kJ/mol
D)PH3(g), +5.4 kJ/mol
E)NH3(g), -46.4 kJ/mol
A)N2O4(g), +9.7 kJ/mol
B)H2S(g), -20.6 kJ/mol
C)N2H4(g), +94.5 kJ/mol
D)PH3(g), +5.4 kJ/mol
E)NH3(g), -46.4 kJ/mol
NH3(g), -46.4 kJ/mol
3
Based on the H°f data given, which of the following compounds is the most unstable?
A)SrO(s), -592.0 kJ/mol
B)CsBr(s), -396 kJ/mol
C)CaF2(s), -1215 kJ/mol
D)NaI(s), -288 kJ/mol
E)KF(s), -568.6 kJ/mol
A)SrO(s), -592.0 kJ/mol
B)CsBr(s), -396 kJ/mol
C)CaF2(s), -1215 kJ/mol
D)NaI(s), -288 kJ/mol
E)KF(s), -568.6 kJ/mol
CaF2(s), -1215 kJ/mol
4
Based on the H°f data given, which compound is the most stable?
A)CO2(g), -393.5 kJ/mol
B)NO2(g), +33.85 kJ/mol
C)NO(g), +90.4 kJ/mol
D)CO(g), -110.5 kJ/mol
E)NH3(g), -46.4 kJ/mol
A)CO2(g), -393.5 kJ/mol
B)NO2(g), +33.85 kJ/mol
C)NO(g), +90.4 kJ/mol
D)CO(g), -110.5 kJ/mol
E)NH3(g), -46.4 kJ/mol

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
5
Given that the first ionization energy of cesium is +376 kJ/mol and the electron affinity of bromine is -325 kJ/mol, calculate E for the reaction, Cs(g)+ Br(g)s Cs+(g)+ Br?(g).
A)+376 kJ/mol
B)+701 kJ/mol
C)+51 kJ/mol
D)-701 kJ/mol
E)-51 kJ/mol
A)+376 kJ/mol
B)+701 kJ/mol
C)+51 kJ/mol
D)-701 kJ/mol
E)-51 kJ/mol

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
6
Lithium fluoride has a lattice energy of -1033 kJ/mol. In the ionic solid AB, A2+ has approximately the same radius as Li+, and B2- has approximately the same radius as F?. What is a reasonable estimate of the lattice energy of AB?
A)About 3 × (-1033 kJ/mol)
B)About -1033 kJ/mol
C)About 4 × (-1033 kJ/mol)
D)About 2 × (-1033 kJ/mol)
E)About 6 × (-1033 kJ/mol)
A)About 3 × (-1033 kJ/mol)
B)About -1033 kJ/mol
C)About 4 × (-1033 kJ/mol)
D)About 2 × (-1033 kJ/mol)
E)About 6 × (-1033 kJ/mol)

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
7
Which ionic solid is likely to have the largest exothermic lattice energy?
A)KCl
B)NaCl
C)LiCl
D)CsCl
E)They all have the same value.
A)KCl
B)NaCl
C)LiCl
D)CsCl
E)They all have the same value.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following solids is likely to have the largest exothermic lattice energy?
A)LiF
B)NaCl
C)AlCl3
D)Al2O3
E)CaCl2
A)LiF
B)NaCl
C)AlCl3
D)Al2O3
E)CaCl2

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
9
Which ionic solid is likely to have the least exothermic lattice energy?
A)KCl
B)NaCl
C)LiCl
D)CsCl
E)RbBr
A)KCl
B)NaCl
C)LiCl
D)CsCl
E)RbBr

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following solids is likely to have the least exothermic lattice energy?
A)LiF
B)NaCl
C)AlCl3
D)Al2O3
E)CaCl2
A)LiF
B)NaCl
C)AlCl3
D)Al2O3
E)CaCl2

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following solids would have the highest melting point?
A)LiF
B)NaCl
C)AlCl3
D)Al2O3
E)CaCl2
A)LiF
B)NaCl
C)AlCl3
D)Al2O3
E)CaCl2

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following statements is true in relation to the formation of the stable ionic compound sodium chloride?Hint: Consider why the sodium to chloride bond occurs.
A)The net absorption of 147 kJ/mol of energy for the formation of the gaseous sodium and chloride ions is mainly responsible for the formation of the stable ionic compound.
B)The lattice energy provides the necessary stabilization energy for the formation of sodium chloride.
C)Sodium chloride is stable because it is formed from the combination of isolated gaseous ions.
D)The first ionization energy of sodium contributes favorably to the overall formation of sodium chloride.
E)The release of energy as the solid sodium chloride is formed leads to an overall increase in the potential energy.
A)The net absorption of 147 kJ/mol of energy for the formation of the gaseous sodium and chloride ions is mainly responsible for the formation of the stable ionic compound.
B)The lattice energy provides the necessary stabilization energy for the formation of sodium chloride.
C)Sodium chloride is stable because it is formed from the combination of isolated gaseous ions.
D)The first ionization energy of sodium contributes favorably to the overall formation of sodium chloride.
E)The release of energy as the solid sodium chloride is formed leads to an overall increase in the potential energy.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
13
For two ions with charges q1 and q2 separated by distance r, the potential energy canbe calculated from Coulomb's law: E = 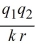 Calculate the energy released when 1 mole NaCl is formed if the constant k = 1.11 × 10-10 C2/J·m, the charge Na+ = +1 e, and the charge on Cl- is -1 e, e = 1.602 × 10-19 C, and
Calculate the energy released when 1 mole NaCl is formed if the constant k = 1.11 × 10-10 C2/J·m, the charge Na+ = +1 e, and the charge on Cl- is -1 e, e = 1.602 × 10-19 C, and 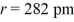
A)+308 kJ/mol
B)+8.20 kJ/mol
C)-297 kJ/mol
D)-494 kJ/mol
E)-371 kJ/mol
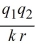 Calculate the energy released when 1 mole NaCl is formed if the constant k = 1.11 × 10-10 C2/J·m, the charge Na+ = +1 e, and the charge on Cl- is -1 e, e = 1.602 × 10-19 C, and
Calculate the energy released when 1 mole NaCl is formed if the constant k = 1.11 × 10-10 C2/J·m, the charge Na+ = +1 e, and the charge on Cl- is -1 e, e = 1.602 × 10-19 C, and 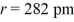
A)+308 kJ/mol
B)+8.20 kJ/mol
C)-297 kJ/mol
D)-494 kJ/mol
E)-371 kJ/mol

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
14
Sodium forms a monatomic ion that has the electron configuration of a noble gas. What is the electron configuration of that noble gas?
A)1s2
B)1s2 2p6
C)1s2 2s2 2p6
D)1s2 2s2 2p6 3s2
E)1s2 2s2 2p6 3s2 3p6
A)1s2
B)1s2 2p6
C)1s2 2s2 2p6
D)1s2 2s2 2p6 3s2
E)1s2 2s2 2p6 3s2 3p6

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
15
Magnesium forms a monatomic ion that has the electron configuration of a noble gas. What is the electron configuration of that noble gas?
A)1s2 2p6
B)1s2 2s2 2p6
C)1s2 2s2 2p6 3s1
D)1s2 2s2 2p6 3s2
E)1s2 2s2 2p6 3s2 3p6
A)1s2 2p6
B)1s2 2s2 2p6
C)1s2 2s2 2p6 3s1
D)1s2 2s2 2p6 3s2
E)1s2 2s2 2p6 3s2 3p6

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
16
Bromine tends to form a monatomic ion that has the electron configuration of a noble gas. What is the symbol of that noble gas?
A)Ne
B)Ar
C)Kr
D)Xe
E)Rn
A)Ne
B)Ar
C)Kr
D)Xe
E)Rn

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
17
Sulfur tends to form a monatomic ion that has the electron configuration of a noble gas. What is the symbol of the noble gas?
A)Ne
B)Ar
C)Kr
D)Xe
E)Rn
A)Ne
B)Ar
C)Kr
D)Xe
E)Rn

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
18
The octet rule is generally followed for cations of elements located where on the periodic table?
A)post transition metals and transition metals
B)Group IA and Group IIA metals
C)Group IVA elements
D)lanthanide elements
E)actinide elements
A)post transition metals and transition metals
B)Group IA and Group IIA metals
C)Group IVA elements
D)lanthanide elements
E)actinide elements

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
19
Which ion of uranium has a noble gas electron configuration?
A)U2+
B)U3+
C)U4+
D)U6+
E)U2-
A)U2+
B)U3+
C)U4+
D)U6+
E)U2-

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
20
Which ion has a noble gas electron configuration?
A)Fe3+
B)Sn2+
C)Ni2+
D)Ti4+
E)Cr3+
A)Fe3+
B)Sn2+
C)Ni2+
D)Ti4+
E)Cr3+

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
21
Which ion has a noble gas electron configuration?
A)Fe3+
B)Ni2+
C)Sc3+
D)V3+
E)Cr3+
A)Fe3+
B)Ni2+
C)Sc3+
D)V3+
E)Cr3+

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
22
Which atom has the same electron configuration as In3+?
A)Te
B)Pd
C)Ir
D)Zn
E)Ga
A)Te
B)Pd
C)Ir
D)Zn
E)Ga

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
23
Give the ground state electron configuration and number of unpaired electrons for Sb3+. Hint: Draw the orbital diagram of Sb3+ to determine unpaired electrons.
A)[Kr] 4d1 5s1; 2 unpaired electrons
B)[Ar]; 1 unpaired electron
C)[Kr] 4d10 5s2; 0 unpaired electrons
D)[Kr] 4d7 5s2; 3 unpaired electrons
E)[Ar] 4d4 5s2; 4 unpaired electrons
A)[Kr] 4d1 5s1; 2 unpaired electrons
B)[Ar]; 1 unpaired electron
C)[Kr] 4d10 5s2; 0 unpaired electrons
D)[Kr] 4d7 5s2; 3 unpaired electrons
E)[Ar] 4d4 5s2; 4 unpaired electrons

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
24
Give the ground state electron configuration and number of unpaired electrons for V3+. Hint: Draw the orbital diagram of V3+ to determine unpaired electrons.
A)[Ar] 3d1; 2 unpaired electrons
B)[Ar]; 1 unpaired electron
C)[Ar] 3d2; 2 unpaired electrons
D)[Ar] 3d3; 3 unpaired electrons
E)[Ar] 3d4; 4 unpaired electrons
A)[Ar] 3d1; 2 unpaired electrons
B)[Ar]; 1 unpaired electron
C)[Ar] 3d2; 2 unpaired electrons
D)[Ar] 3d3; 3 unpaired electrons
E)[Ar] 3d4; 4 unpaired electrons

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
25
Which metal ion is expected to have the electron configuration [Kr]4d4? Hint: Remember that positive ions lose electrons.
A)Mn2+
B)Ru2+
C)Zr2+
D)Mo2+
E)Sr2+
A)Mn2+
B)Ru2+
C)Zr2+
D)Mo2+
E)Sr2+

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
26
Which element below has three valence electrons in its Lewis symbol?
A)gallium
B)fluorine
C)iron
D)nickel
E)sulfur
A)gallium
B)fluorine
C)iron
D)nickel
E)sulfur

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
27
Which species below has eight valence electrons in its Lewis symbol?
A)Ar+
B)F+
C)Mg+
D)S2-
E)Si
A)Ar+
B)F+
C)Mg+
D)S2-
E)Si

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
28
Which species below has the lowest number of valence electrons in its Lewis symbol?
A)Ar+
B)Ga+
C)Mg2+
D)S2-
E)F?
A)Ar+
B)Ga+
C)Mg2+
D)S2-
E)F?

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
29
Which element below includes six electrons in its Lewis symbol?
A)Cr
B)Ni
C)Ba
D)Se
E)As
A)Cr
B)Ni
C)Ba
D)Se
E)As

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
30
Which species has the most valence electrons in its Lewis symbol?
A)Ar+
B)Ga+
C)Ba2+
D)Br
E)O2?
A)Ar+
B)Ga+
C)Ba2+
D)Br
E)O2?

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
31
The atoms in the nitrogen molecule, N2, are held together by Hint: Draw the Lewis structure of the molecule.
A)a single covalent bond.
B)a double covalent bond.
C)a triple covalent bond.
D)an ionic bond.
E)a magnetic dipole bond.
A)a single covalent bond.
B)a double covalent bond.
C)a triple covalent bond.
D)an ionic bond.
E)a magnetic dipole bond.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
32
The atoms in the oxygen molecule, O2, are held together by Hint: Draw the Lewis structure of the molecule.
A)a single covalent bond.
B)a double covalent bond.
C)a triple covalent bond.
D)an ionic bond.
E)a magnetic dipole bond.
A)a single covalent bond.
B)a double covalent bond.
C)a triple covalent bond.
D)an ionic bond.
E)a magnetic dipole bond.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
33
A covalent bond is characterized by two properties. These are
A)the energy of the bond and the location of the electron pair in the bond.
B)the energy of the separated atoms and the energy of the new bond.
C)the bond length and the polarity of the covalent bond.
D)the bond length and the bond energy.
E)the energy released when a bond forms, and the electronegativities of the two atoms.
A)the energy of the bond and the location of the electron pair in the bond.
B)the energy of the separated atoms and the energy of the new bond.
C)the bond length and the polarity of the covalent bond.
D)the bond length and the bond energy.
E)the energy released when a bond forms, and the electronegativities of the two atoms.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
34
The Lewis symbol for the carbon atom has ________ valence electrons. The number of covalent bonds that carbon usually forms to complete its valence shell and obey the octet rule is ________.
A)4, 1
B)4, 2
C)2, 4
D)4, 3
E)4, 4
A)4, 1
B)4, 2
C)2, 4
D)4, 3
E)4, 4

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
35
The Lewis symbol for the nitrogen atom has ________ valence electrons. The number of covalent bonds that nitrogen usually forms to complete its valence shell and obey the octet rule is ________.
A)5, 1
B)5, 2
C)3, 4
D)5, 3
E)5, 4
A)5, 1
B)5, 2
C)3, 4
D)5, 3
E)5, 4

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
36
Which statement below is true?Hint: Consider how properties of a covalent bond are measured.
A)The two electrons in a single covalent bond must be paired as required by the Pauli exclusion principle.
B)One mole of hydrogen atoms is more stable than one mole of hydrogen molecules.
C)The buildup of electron density between two atoms repels each nucleus, making them less stable.
D)The bond energy is the minimum energy required to bring about pairing of the electrons in a covalent bond.
E)As the distance between the nuclei decreases when forming a covalent bond, there is a corresponding decrease in the probability of finding both electrons near either nucleus.
A)The two electrons in a single covalent bond must be paired as required by the Pauli exclusion principle.
B)One mole of hydrogen atoms is more stable than one mole of hydrogen molecules.
C)The buildup of electron density between two atoms repels each nucleus, making them less stable.
D)The bond energy is the minimum energy required to bring about pairing of the electrons in a covalent bond.
E)As the distance between the nuclei decreases when forming a covalent bond, there is a corresponding decrease in the probability of finding both electrons near either nucleus.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
37
Which bond is the most polar?
A)H-C
B)H-Cl
C)H-P
D)H-S
E)H-Se
A)H-C
B)H-Cl
C)H-P
D)H-S
E)H-Se

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
38
Which bond is the most polar?
A)H-C
B)H-S
C)H-P
D)H-O
E)H-Se
A)H-C
B)H-S
C)H-P
D)H-O
E)H-Se

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
39
Which bond is the most polar?Hint: Consider the electronegativity values of each element.
A)Al-I
B)Si-I
C)Al-Cl
D)Si-Cl
E)Si-P
A)Al-I
B)Si-I
C)Al-Cl
D)Si-Cl
E)Si-P

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
40
Which bond is the most polar?Hint: Consider the electronegativity values of each element.
A)Br-Br
B)S-O
C)C-P
D)C-O
E)B-O
A)Br-Br
B)S-O
C)C-P
D)C-O
E)B-O

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
41
Which bond is the most polar?Hint: Consider the electronegativity values of each element.
A)B-C
B)C-N
C)C-O
D)Si-O
E)C-C
A)B-C
B)C-N
C)C-O
D)Si-O
E)C-C

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
42
Arrange the following in terms of increasing electronegativity values.Ga, P, As, Ge
A)Ga < Ge < As < P
B)Ge < Ga < As < P
C)P < Ge < Ga < As
D)P < As < Ge < Ga
E)As < Ge < As
A)Ga < Ge < As < P
B)Ge < Ga < As < P
C)P < Ge < Ga < As
D)P < As < Ge < Ga
E)As < Ge < As

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
43
Based on electronegativity considerations, which species is predicted to be the strongest oxidizing agent?
A)Ne
B)Kr
C)Br2
D)Cl2
E)S
A)Ne
B)Kr
C)Br2
D)Cl2
E)S

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
44
Arrange the following in terms of decreasing electronegativity values.P, O, As, S Hint: Remember to refer to your periodic table for trends.
A)P > O > As > S
B)As > P > S > O
C)S > O > P > As
D)As > S > P > O
E)O > S > P > As
A)P > O > As > S
B)As > P > S > O
C)S > O > P > As
D)As > S > P > O
E)O > S > P > As

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
45
In the chlorine monoxide molecule, chlorine has a charge of +0.167 e-. If the bond length is 154.6 pm, calculate the dipole moment of the molecule in debyes. Hint: Watch your units carefully with this problem.
A)3.11 D
B)2.30 D
C)0.167 D
D)1.24 D
E)1.65 D
A)3.11 D
B)2.30 D
C)0.167 D
D)1.24 D
E)1.65 D

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
46
In the nitrogen monoxide molecule, the dipole moment is 0.16 D and the bond length is 115 pm. Determine the sign and magnitude of the charge on the oxygen atom. Hint: Watch your units carefully with this problem.
A)-1.3 e-
B)-0.029 e-
C)-0.097 e-
D)-0.72 e-
E)+1.3 e-
A)-1.3 e-
B)-0.029 e-
C)-0.097 e-
D)-0.72 e-
E)+1.3 e-

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
47
Based on the "best"Lewis structure after applying formal charge considerations, how many non-bonding valence electrons are around the nitrogen atom in the nitrate ion?
A)0
B)2
C)4
D)6
E)8
A)0
B)2
C)4
D)6
E)8

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
48
Draw the Lewis structure for HClO2 from the skeletal structure presented below. If the valence shells are filled to the usual limit (a maximum of 8), how many nonbonding valence electrons are in the molecule? 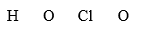
A)14
B)16
C)18
D)20
E)26
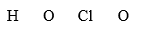
A)14
B)16
C)18
D)20
E)26

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
49
Draw the Lewis structure for HNO2. If the valence shells are filled to the usual limit (a maximum of 8), how many nonbonding valence electrons are in the molecule?
A)0
B)2
C)6
D)10
E)14
A)0
B)2
C)6
D)10
E)14

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
50
Draw a Lewis structure for CH2Cl2. Based on this Lewis structure, the calculated value for the formal charge on the carbon atom is
A)0.
B)4+.
C)2+.
D)2-.
E)4-.
A)0.
B)4+.
C)2+.
D)2-.
E)4-.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
51
Draw a Lewis structure for NH4+. Based on this Lewis structure, the calculated value for the formal charge on the nitrogen atom is
A)0.
B)4+.
C)1+.
D)1-.
E)4- .
A)0.
B)4+.
C)1+.
D)1-.
E)4- .

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
52
When the fluoride ion reacts with a BF3 molecule (a molecule in which there are no multiple bonds), an ion is formed in which the boron atom is the central atom. The bond between the boron trifluoride and the fluoride ion is
A)an ionic bond.
B)a regular covalent bond, where both species contribute 1 electron to the bond.
C)a coordinate covalent bond.
D)a resonance hybrid bond.
E)a bond where two atoms share one electron instead of two.
A)an ionic bond.
B)a regular covalent bond, where both species contribute 1 electron to the bond.
C)a coordinate covalent bond.
D)a resonance hybrid bond.
E)a bond where two atoms share one electron instead of two.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
53
How many coordinate covalent bonds are formed in the reaction between H+ and NH3 and what is the formula of the only product?
A)0, NH3
B)1, NH4+
C)2, NH4+
D)3, N2H4+
E)4, N2H5+
A)0, NH3
B)1, NH4+
C)2, NH4+
D)3, N2H4+
E)4, N2H5+

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
54
How many coordinate covalent bonds are formed in this product in the reaction between F- and BF3 and what is the formula of the product?
A)BF3, 0
B)BF4-, 1
C)BF4-, 2
D)BF52-, 3
E)B2F5-, 4
A)BF3, 0
B)BF4-, 1
C)BF4-, 2
D)BF52-, 3
E)B2F5-, 4

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
55
When the hydrogen ion combines with a water molecule, a coordinate covalent bond is formed because
A)a monatomic species reacts with a molecular substance.
B)both electrons are supplied by the oxygen atom of the water molecule.
C)a nonpolar bond is formed.
D)a polyatomic species is formed.
E)a bond is formed where two atoms share one electron instead of two.
A)a monatomic species reacts with a molecular substance.
B)both electrons are supplied by the oxygen atom of the water molecule.
C)a nonpolar bond is formed.
D)a polyatomic species is formed.
E)a bond is formed where two atoms share one electron instead of two.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
56
Draw the Lewis structure for hydrogen peroxide, H2O2. Based on this structure, how many polar bonds and non-polar bonds are present?
A)3 polar bonds and no non-polar bonds
B)2 polar bonds and 1 non-polar bond
C)1 polar bond and 2 non-polar bonds
D)no polar bonds and 3 non-polar bonds
E)2 polar bonds and 2 non-polar bonds
A)3 polar bonds and no non-polar bonds
B)2 polar bonds and 1 non-polar bond
C)1 polar bond and 2 non-polar bonds
D)no polar bonds and 3 non-polar bonds
E)2 polar bonds and 2 non-polar bonds

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
57
How many lone pairs of electrons are in the nitric oxide molecule?
A)2
B)1
C)0
D)3
E)4
A)2
B)1
C)0
D)3
E)4

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
58
The formal charge on the nitrogen atom in the nitrate ion (NO3-)is
A)3-
B)0
C)1+
D)3+
E)5+
A)3-
B)0
C)1+
D)3+
E)5+

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
59
The formal charge on the carbon atom in the carbonate (CO32-)ion is
A)2-.
B)0.
C)1+.
D)2+.
E)4+.
A)2-.
B)0.
C)1+.
D)2+.
E)4+.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
60
Based on the "best"Lewis structure after applying formal charge considerations, how many non-bonding valence electrons are around the carbon atom in the CO molecule?
A)0
B)2
C)4
D)6
E)8
A)0
B)2
C)4
D)6
E)8

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
61
Based on the "best"Lewis structure after applying formal charge considerations, how many non-bonding valence electrons are around the nitrogen atom in the nitrite ion (NO2-)?
A)0
B)2
C)4
D)6
E)8
A)0
B)2
C)4
D)6
E)8

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
62
Based on the "best"Lewis structure after applying formal charge considerations, how many non-bonding valence electrons are around the nitrogen atom in the HCN molecule?
A)0
B)1
C)2
D)4
E)6
A)0
B)1
C)2
D)4
E)6

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
63
Draw the Lewis structure for HClO3. If the valence shells are filled to the usual limit (a maximum of 8), what is the formal charge on the chlorine atom? Hint: Do not forget about double or triple bonds potentially being in the Lewis structure.
A)-1
B)0
C)+1
D)+2
E)+3
A)-1
B)0
C)+1
D)+2
E)+3

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
64
Draw the Lewis structure for H2SeO3. If the valence shells are filled to the usual limit (a maximum of 8), how many nonbonding valence electrons are there in the molecule? Hint: Do not forget about double or triple bonds potentially being in the Lewis structure.
A)14
B)16
C)18
D)20
E)28
A)14
B)16
C)18
D)20
E)28

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
65
Draw a Lewis structure for H3C-NH2. Based on this Lewis structure, the calculated value for the formal charge on the nitrogen atom is Hint: Do not forget about double or triple bonds potentially being in the Lewis structure.
A)2-.
B)3+.
C)3-.
D)2+.
E)0.
A)2-.
B)3+.
C)3-.
D)2+.
E)0.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
66
Draw the Lewis structure for the H2CO molecule. Based on this structure, how many polar bonds and non-polar bonds are present? Hint: Do not forget about double or triple bonds potentially being in the Lewis structure.
A)3 polar bonds and no non-polar bonds
B)2 polar bonds and 1 non-polar bond
C)1 polar bond and 2 non-polar bonds
D)no polar bonds and 3 non-polar bonds
E)2 polar bonds and 2 non-polar bonds
A)3 polar bonds and no non-polar bonds
B)2 polar bonds and 1 non-polar bond
C)1 polar bond and 2 non-polar bonds
D)no polar bonds and 3 non-polar bonds
E)2 polar bonds and 2 non-polar bonds

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
67
Draw the Lewis structure for the F2CO molecule. Based on this structure, how many polar bonds and non-polar bonds are present? Hint: Do not forget about double or triple bonds potentially being in the Lewis structure.
A)3 polar bonds and no non-polar bonds
B)2 polar bonds and 1 non-polar bond
C)1 polar bond and 2 non-polar bonds
D)no polar bonds and 3 non-polar bonds
E)2 polar bonds and 2 non-polar bonds
A)3 polar bonds and no non-polar bonds
B)2 polar bonds and 1 non-polar bond
C)1 polar bond and 2 non-polar bonds
D)no polar bonds and 3 non-polar bonds
E)2 polar bonds and 2 non-polar bonds

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
68
Which species has the same bond order as the carbon monoxide molecule?
A)ClF
B)CO32-
C)CO2
D)NO+
E)O3
A)ClF
B)CO32-
C)CO2
D)NO+
E)O3

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
69
The metaphosphate ion, PO3-, is the structural analog of the NO3- ion with respect to the arrangement of the atoms in the ion. After drawing the "best"Lewis structure for the metaphosphate ion based on formal charge considerations, what is the formal charge on the phosphorus atom? Hint: Do not forget about double or triple bonds potentially being in the Lewis structure.
A)1-
B)0
C)1+
D)2+
E)5+
A)1-
B)0
C)1+
D)2+
E)5+

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
70
Draw the Lewis structure for H2SeO4. If the valence shells are filled to the usual limit (a maximum of 8), what is the formal charge on the Se? Hint: Do not forget about double or triple bonds potentially being in the Lewis structure.
A)2-
B)1-
C)0
D)1+
E)2+
A)2-
B)1-
C)0
D)1+
E)2+

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
71
Draw the Lewis structure for H2SO4. Experimentally, it is known that the S-O bonds are shorter than the S-OH bonds. The likely explanation for this is Hint: Consider the number of bonded electrons in each bond.
A)the electrons in the S-OH bonds are not equally shared.
B)the sulfur atom is not as electronegative as the oxygen atom.
C)the hydrogen atom is weakly bonded to the other S atom.
D)there are no lone pairs on the hydrogen atom.
E)the S-O bond has a greater bond order than the S-OH bond.
A)the electrons in the S-OH bonds are not equally shared.
B)the sulfur atom is not as electronegative as the oxygen atom.
C)the hydrogen atom is weakly bonded to the other S atom.
D)there are no lone pairs on the hydrogen atom.
E)the S-O bond has a greater bond order than the S-OH bond.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
72
Draw the Lewis structure for HClO3. If the valence shells are filled to the usual limit (a maximum of 8), what is the sum of the absolute values of all the formal charges in the molecule? Hint: Minimize formal charge to ensure you have the most stable or "best"Lewis structure.
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
73
Draw a Lewis structure for CH3NO2. Based on the Lewis structure, what is the formal charge on the nitrogen atom for the most favorable structure? Hint: Minimize formal charge to ensure you have the most stable or "best"Lewis structure.
A)-2
B)-1
C)0
D)+1
E)+2
A)-2
B)-1
C)0
D)+1
E)+2

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
74
Draw the "best"Lewis structure for sulfur trioxide based on formal charge considerations. The number of resonance structures is
A)1 (no resonance structures).
B)2.
C)3.
D)4.
E)5.
A)1 (no resonance structures).
B)2.
C)3.
D)4.
E)5.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
75
Based on the "best"Lewis structure from formal charge considerations, how many resonance structures, if any, can be drawn for the PO43- ion?
A)1 (no resonance structures)
B)2
C)3
D)4
E)5
A)1 (no resonance structures)
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
76
The metaphosphate ion, PO3-, is the structural analog of the NO3- ion with respect to the arrangement of the atoms in the ion. After drawing the "best"Lewis structure for the metaphosphate ion based on formal charge considerations, what is the number of resonance structures for this "best"Lewis structure? Hint: Use formal charge to help determine how many realistic resonance structures there are for the metaphosphate ion.
A)1 (no resonance structures)
B)2
C)3
D)4
E)5
A)1 (no resonance structures)
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
77
How many resonance structures, if any, can be drawn for the N2O5 molecule? Hint: Consider formal charge when determining if the resonance structure is valid.
A)1 (no resonance structures)
B)2
C)3
D)4
E)5
A)1 (no resonance structures)
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
78
The chlorite ion has a Lewis structure that is based on the skeletal structure shown below: O=Cl -O
Draw the Lewis structure minimizing the formal charges present in the structure. Based on this structure, the chlorite ion has ,Hint: Consider all possible resonance structures when working with formal charge.
A)two single bonds, the sum of absolute values of formal charges = 3, and no resonance hybrids.
B)one single and one double bond, sum of absolute values of formal charges = 5, and two contributing resonance hybrids.
C)one single and one double bond, sum of absolute values of formal charges = 3, and two contributing resonance hybrids.
D)two double bonds, sum of absolute values of formal charges = 1, and no resonance hybrids.
E)one single and one double bond, sum of absolute values of formal charges = 1, and two contributing resonance hybrids.
Draw the Lewis structure minimizing the formal charges present in the structure. Based on this structure, the chlorite ion has ,Hint: Consider all possible resonance structures when working with formal charge.
A)two single bonds, the sum of absolute values of formal charges = 3, and no resonance hybrids.
B)one single and one double bond, sum of absolute values of formal charges = 5, and two contributing resonance hybrids.
C)one single and one double bond, sum of absolute values of formal charges = 3, and two contributing resonance hybrids.
D)two double bonds, sum of absolute values of formal charges = 1, and no resonance hybrids.
E)one single and one double bond, sum of absolute values of formal charges = 1, and two contributing resonance hybrids.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
79
The carbonate ion has the skeletal structure as shown below. Complete the Lewis structure by filling in the bonds and the remaining valence electrons which are not involved in bonds. Which statement made about the carbonate ion is true? 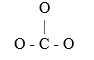 Based on its Lewis structure, the carbonate ion should have ________ resonance hybrids and ________ atoms with formal charges on them in its structure. Hint: Consider the possible places the double bond could go when determining resonance of carbonate.
Based on its Lewis structure, the carbonate ion should have ________ resonance hybrids and ________ atoms with formal charges on them in its structure. Hint: Consider the possible places the double bond could go when determining resonance of carbonate.
A)2, 2
B)3, 1
C)3, 2
D)2, 1
E)no, 2
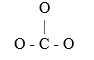 Based on its Lewis structure, the carbonate ion should have ________ resonance hybrids and ________ atoms with formal charges on them in its structure. Hint: Consider the possible places the double bond could go when determining resonance of carbonate.
Based on its Lewis structure, the carbonate ion should have ________ resonance hybrids and ________ atoms with formal charges on them in its structure. Hint: Consider the possible places the double bond could go when determining resonance of carbonate.A)2, 2
B)3, 1
C)3, 2
D)2, 1
E)no, 2

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
80
Draw the Lewis structure of CH3NO2.Based on its structure, the nitromethane molecule should have ________ resonance hybrids and ________ atoms with formal charges on them. Hint: Consider possible double and triple bonds in the structure and be sure to minimize formal charge.
A)no, 2
B)no, 3
C)2, 2
D)2, 3
E)3, 2
A)no, 2
B)no, 3
C)2, 2
D)2, 3
E)3, 2

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck


