Deck 14: Kinetics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/79
Play
Full screen (f)
Deck 14: Kinetics
1
Which of the following experimental conditions describe a reaction which is kinetically controlled?
A) G = +100 kJ/molrxn; there is no observable reaction when reactants are mixed.
B) G = - 100 kJ/molrxn; there is no observable reaction when reactants are mixed.
C) G = +100 kJ/molrxn; the reaction proceeds rapidly when reactants are mixed.
D) G = - 100 kJ/molrxn; the reaction proceeds rapidly when reactants are mixed.
E) These conditions do not describe kinetic control.
A) G = +100 kJ/molrxn; there is no observable reaction when reactants are mixed.
B) G = - 100 kJ/molrxn; there is no observable reaction when reactants are mixed.
C) G = +100 kJ/molrxn; the reaction proceeds rapidly when reactants are mixed.
D) G = - 100 kJ/molrxn; the reaction proceeds rapidly when reactants are mixed.
E) These conditions do not describe kinetic control.
G = - 100 kJ/molrxn; there is no observable reaction when reactants are mixed.
2
Use the following table to determine the average rate during the period 800 to 1200 seconds for the decomposition of H2O2. 
A) 8.0 x 10 - 4 M/sec
B) 8.2 x 10 - 4 M/s
C) 1.2 x 10 - 3 M/s
D) 1.6 x 10 - 3 M/s
E) 2.4 x 10 - 3 M/s

A) 8.0 x 10 - 4 M/sec
B) 8.2 x 10 - 4 M/s
C) 1.2 x 10 - 3 M/s
D) 1.6 x 10 - 3 M/s
E) 2.4 x 10 - 3 M/s
8.0 x 10 - 4 M/sec
3
Determine the units for the rate constant, k, in the following rate expression: rate = k(A)2(B).
A) s - 1
B) M/s
C) M2/s
D) M3/s
E) M - 2 s - 1
A) s - 1
B) M/s
C) M2/s
D) M3/s
E) M - 2 s - 1
M - 2 s - 1
4
What will be the units of the rate constant, k, for the following reaction?
2 NO(g) + 2 H2(g) N2(g) + 2 H2O(g) rate = k (NO)2(H2)
A) s - 1
B) M/s
C) M - 1 s - 1
D) M - 2 s - 1
E) M2 s - 1
2 NO(g) + 2 H2(g) N2(g) + 2 H2O(g) rate = k (NO)2(H2)
A) s - 1
B) M/s
C) M - 1 s - 1
D) M - 2 s - 1
E) M2 s - 1

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
5
The disproportionation of NO to N2O and NO2 is third order in NO.
3 NO(g) F N2O(g) + NO2(g) rate = k (NO)3
What are the units for the rate constant, k?
A) M3/s
B) M2/s2
C) M - 3 s - 1
D) M - 2 s - 2
E) none of these
3 NO(g) F N2O(g) + NO2(g) rate = k (NO)3
What are the units for the rate constant, k?
A) M3/s
B) M2/s2
C) M - 3 s - 1
D) M - 2 s - 2
E) none of these

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
6
Nitrogen reacts with hydrogen to form ammonia by the following reaction:
N2(g) + 3 H2(g) 2 NH3(g)
If the rate of consumption of hydrogen is 0.060 M/s, what is the rate at which N2 is consumed?
A) 0.010 M/s
B) 0.020 M/s
C) 0.030 M/s
D) 0.060 M/s
E) 0.180 M/s
N2(g) + 3 H2(g) 2 NH3(g)
If the rate of consumption of hydrogen is 0.060 M/s, what is the rate at which N2 is consumed?
A) 0.010 M/s
B) 0.020 M/s
C) 0.030 M/s
D) 0.060 M/s
E) 0.180 M/s

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
7
Which equation describes the relationship between the rates at which NO is consumed and N2 is produced in the following reaction?
2 NO(g) + 2 H2(g) N2(g) + 2 H2O(g)
A) rate N2 = rate NO
B) rate N2 = 2(rate NO)
C) 2(rate N2) = rate NO
D) (rate N2)2 = rate NO
E) rate N2 = (rate NO)2
2 NO(g) + 2 H2(g) N2(g) + 2 H2O(g)
A) rate N2 = rate NO
B) rate N2 = 2(rate NO)
C) 2(rate N2) = rate NO
D) (rate N2)2 = rate NO
E) rate N2 = (rate NO)2

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
8
The reaction 2A B is second order with respect to A. If the concentration of A is decreased by half, what will happen to the rate of the reaction?
A) The rate will double.
B) The rate will decrease by half.
C) The rate will remain constant.
D) The rate will increase by ln(A).
E) The rate will decrease by a factor of 4.
A) The rate will double.
B) The rate will decrease by half.
C) The rate will remain constant.
D) The rate will increase by ln(A).
E) The rate will decrease by a factor of 4.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
9
For the reaction
3 N2O(g) + C2H2(g) 3 N2(g) + 2 CO(g) + H2O(g)
If water is produced at the rate of 0.10 M/s, what is the rate of production of N2?
A) 0.10 M/s
B) 0.033 M/s
C) 0.30 M/s
D) 0.20 M/s
E) none of these
3 N2O(g) + C2H2(g) 3 N2(g) + 2 CO(g) + H2O(g)
If water is produced at the rate of 0.10 M/s, what is the rate of production of N2?
A) 0.10 M/s
B) 0.033 M/s
C) 0.30 M/s
D) 0.20 M/s
E) none of these

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following equations correctly describes the relationship between the instantaneous rate at which NO2 and Cl2 are consumed in the following reaction?
2 NO2(g) + Cl2(g) 2 NO2Cl(g)
A) -d(NO2)/dt = 1/2 [-d(Cl2)/dt]
B) -d(NO2)/dt = 1/2 [d(Cl2)/dt]
C) -d(NO2)/dt = -d(Cl2)/dt
D) -d(NO2)/dt = 2 [-d(Cl2)/dt]
E) -d(NO2)/dt = 2 [d(Cl2)/dt]
2 NO2(g) + Cl2(g) 2 NO2Cl(g)
A) -d(NO2)/dt = 1/2 [-d(Cl2)/dt]
B) -d(NO2)/dt = 1/2 [d(Cl2)/dt]
C) -d(NO2)/dt = -d(Cl2)/dt
D) -d(NO2)/dt = 2 [-d(Cl2)/dt]
E) -d(NO2)/dt = 2 [d(Cl2)/dt]

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
11
In the following reaction
2 VO43-(aq) + 3 Zn(s) + 16 H+(aq) 2 V2+(aq) + 3 Zn2+(aq) + 8 H2O(l)
The initial rate of disappearance of VO43- was found to be 0.56 M/s. What is the initial rate of appearance of Zn2+?
A) -0.56 M/s
B) 0.37 M/s
C) 0.56 M/s
D) 0.84 M/s
E) 1.12 M/s
2 VO43-(aq) + 3 Zn(s) + 16 H+(aq) 2 V2+(aq) + 3 Zn2+(aq) + 8 H2O(l)
The initial rate of disappearance of VO43- was found to be 0.56 M/s. What is the initial rate of appearance of Zn2+?
A) -0.56 M/s
B) 0.37 M/s
C) 0.56 M/s
D) 0.84 M/s
E) 1.12 M/s

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
12
The instantaneous rate of appearance of water from the reaction
4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g)
At some moment in time is 21.3 mmHg/min. What is the instantaneous rate of disappearance of NH3 at the same moment in time?
A) 10.7 mmHg/min
B) 14.2 mmHg/min
C) 21.3 mmHg/min
D) 32.0 mmHg/min
E) none of these
4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g)
At some moment in time is 21.3 mmHg/min. What is the instantaneous rate of disappearance of NH3 at the same moment in time?
A) 10.7 mmHg/min
B) 14.2 mmHg/min
C) 21.3 mmHg/min
D) 32.0 mmHg/min
E) none of these

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
13
When NH3 is treated with O2 at elevated temperatures, the rate of disappearance of NH3 is found to be 3.5 x 10-2 M s-1. The equation for the reaction is
4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g)
Calculate the rate of appearance of NO and H2O.
4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g)
Calculate the rate of appearance of NO and H2O.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
14
For which reactant or product in the following reaction will the rate of change of concentration with time be the largest?
6 Fe2+ + Cr2O72- + 14 H+ 2 Cr3+ + 6 Fe3+ + 7 H2O
A) Cr3+
B) Cr2O72-
C) Fe2+
D) H+
E) H2O
6 Fe2+ + Cr2O72- + 14 H+ 2 Cr3+ + 6 Fe3+ + 7 H2O
A) Cr3+
B) Cr2O72-
C) Fe2+
D) H+
E) H2O

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
15
What is the correct rate law for the following reaction?
2 NO2(g) + F2(g) 2 NO2F(g)
A) rate = kf(NO)2
B) rate = kf(NO2)2(F2)
C) rate = -kf(NO2)2(F2)
D) rate = -kf(F2)
E) Not enough information is available to determine the rate law.
2 NO2(g) + F2(g) 2 NO2F(g)
A) rate = kf(NO)2
B) rate = kf(NO2)2(F2)
C) rate = -kf(NO2)2(F2)
D) rate = -kf(F2)
E) Not enough information is available to determine the rate law.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following affect whether a collision between molecules will result in a chemical reaction?
A) change in enthalpy for the reaction
B) orientation of the molecules
C) energy of the collision
D) 'b' and 'c' only
E) all of these
A) change in enthalpy for the reaction
B) orientation of the molecules
C) energy of the collision
D) 'b' and 'c' only
E) all of these

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
17
Sum the following elementary steps in a mechanism to determine the stoichiometry of the overall reaction.
Cl2(g) 2Cl(g)
Cl(g) + CO(g) COCl(g)
COCl(g) + Cl(g) COCl2(g)
A) Cl2(g) + 2 CO(g) + 2 Cl(g) 2 COCl2(g)
B) 2 Cl2(g) + CO(g) COCl2(g) + 2 Cl(g)
C) 2 Cl(g) + CO(g) COCl2(g)
D) Cl2(g) + CO(g) + Cl(g) COCl2(g) + COCl(g)
E) none of these
Cl2(g) 2Cl(g)
Cl(g) + CO(g) COCl(g)
COCl(g) + Cl(g) COCl2(g)
A) Cl2(g) + 2 CO(g) + 2 Cl(g) 2 COCl2(g)
B) 2 Cl2(g) + CO(g) COCl2(g) + 2 Cl(g)
C) 2 Cl(g) + CO(g) COCl2(g)
D) Cl2(g) + CO(g) + Cl(g) COCl2(g) + COCl(g)
E) none of these

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
18
The reaction between chloroform (CHCl3) and chlorine gas (Cl2) proceeds by the following mechanism.
Cl2 2 Cl
CHCl3 + Cl CCl3 + HCl
CCl3 + Cl CCl4
Which of the following substances are intermediates in the above mechanism?
A) Cl
B) CCl3
C) CCl4
D) HCl
E) Cl & CCl3
Cl2 2 Cl
CHCl3 + Cl CCl3 + HCl
CCl3 + Cl CCl4
Which of the following substances are intermediates in the above mechanism?
A) Cl
B) CCl3
C) CCl4
D) HCl
E) Cl & CCl3

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
19
The overall rate of a chemical reaction is determined by
A) the fastest step in the reaction mechanism.
B) the first step in the reaction mechanism.
C) the slowest step in the reaction mechanism.
D) the last step in the reaction mechanism.
E) G for the overall reaction.
A) the fastest step in the reaction mechanism.
B) the first step in the reaction mechanism.
C) the slowest step in the reaction mechanism.
D) the last step in the reaction mechanism.
E) G for the overall reaction.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following rate laws suggests that the reaction probably occurs in a single step?
A)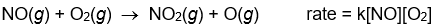
B)
C)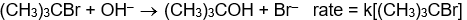
D)
A)
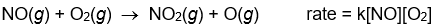
B)

C)
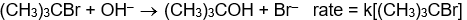
D)


Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
21
Use the rate laws given below to determine which of the following reactions most likely occurs in a single step.
A)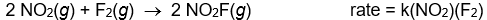
B)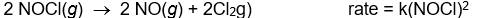
C)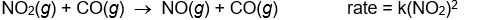
D)
E)
A)
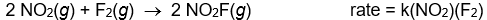
B)
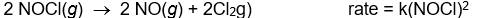
C)
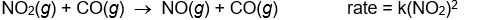
D)

E)


Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
22
refer to the reaction:
NO(g) + O3(g) NO2(g) + O2(g)
for which the following rate data were obtained.

-The rate law for the reaction would be:
A) zero-order in NO
B) first-order in NO
C) second-order in NO
D) third-order in NO
E) none of the above
NO(g) + O3(g) NO2(g) + O2(g)
for which the following rate data were obtained.

-The rate law for the reaction would be:
A) zero-order in NO
B) first-order in NO
C) second-order in NO
D) third-order in NO
E) none of the above

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
23
refer to the reaction:
NO(g) + O3(g) NO2(g) + O2(g)
for which the following rate data were obtained.

-If these data were obtained by watching the rate at which NO disappears, what would be the initial instantaneous rate of disappearance of O3 in
Trial 3?
A) 1.6 x 10-5 M/s
B) 3.2 x 10-5 M/s
C) 4.8 x 10-5 M/s
D) 9.6 x 10-5 M s
E) 14.4 x 10-5 M/s
NO(g) + O3(g) NO2(g) + O2(g)
for which the following rate data were obtained.

-If these data were obtained by watching the rate at which NO disappears, what would be the initial instantaneous rate of disappearance of O3 in
Trial 3?
A) 1.6 x 10-5 M/s
B) 3.2 x 10-5 M/s
C) 4.8 x 10-5 M/s
D) 9.6 x 10-5 M s
E) 14.4 x 10-5 M/s

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
24
refer to the reaction:
NO(g) + O3(g) NO2(g) + O2(g)
for which the following rate data were obtained.

-If a trial was run in which the initial (NO) was 3.15 x 10-6 M and the initial (O3) was 3.15 x 10-6 M, the initial rate of reaction would be:
A) 1.6 x 10-5 M/s
B) 3.2 x 10-5 M/s
C) 3.6 x 10-5 M/s
D) 4.8 x 10-5 M/s
E) 7.2 x 10-5 M/s
NO(g) + O3(g) NO2(g) + O2(g)
for which the following rate data were obtained.

-If a trial was run in which the initial (NO) was 3.15 x 10-6 M and the initial (O3) was 3.15 x 10-6 M, the initial rate of reaction would be:
A) 1.6 x 10-5 M/s
B) 3.2 x 10-5 M/s
C) 3.6 x 10-5 M/s
D) 4.8 x 10-5 M/s
E) 7.2 x 10-5 M/s

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
25
This data applies to the next three problems.
The initial rate of disappearance of bromine (Br2) for the reaction shown below was measured for several different concentrations of bromine, CH3COCH3, and H+ ions. (Note that H+ is a catalyst in this reaction - it participates in the reaction but is not itself consumed)
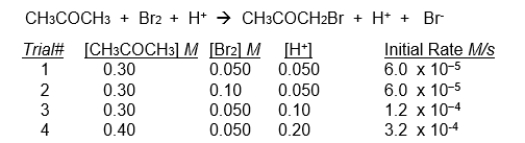
-The rate law for this reaction would be:
A) zero-order in H+ and first-order in Br2
B) first-order in H+ and first-order in Br2
C) first-order in H+ and zero-order in Br2
D) second-order in H+ and zero-order in Br2
E) none of the above.
The initial rate of disappearance of bromine (Br2) for the reaction shown below was measured for several different concentrations of bromine, CH3COCH3, and H+ ions. (Note that H+ is a catalyst in this reaction - it participates in the reaction but is not itself consumed)

-The rate law for this reaction would be:
A) zero-order in H+ and first-order in Br2
B) first-order in H+ and first-order in Br2
C) first-order in H+ and zero-order in Br2
D) second-order in H+ and zero-order in Br2
E) none of the above.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
26
This data applies to the next three problems.
The initial rate of disappearance of bromine (Br2) for the reaction shown below was measured for several different concentrations of bromine, CH3COCH3, and H+ ions. (Note that H+ is a catalyst in this reaction - it participates in the reaction but is not itself consumed)

-The rate law for this reaction would be:
A) zero-order in CH3COCH3
B) half-order in CH3COCH3
C) first-order in CH3COCH3
D) second-order in CH3COCH3
E) none of the above.
The initial rate of disappearance of bromine (Br2) for the reaction shown below was measured for several different concentrations of bromine, CH3COCH3, and H+ ions. (Note that H+ is a catalyst in this reaction - it participates in the reaction but is not itself consumed)

-The rate law for this reaction would be:
A) zero-order in CH3COCH3
B) half-order in CH3COCH3
C) first-order in CH3COCH3
D) second-order in CH3COCH3
E) none of the above.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
27
This data applies to the next three problems.
The initial rate of disappearance of bromine (Br2) for the reaction shown below was measured for several different concentrations of bromine, CH3COCH3, and H+ ions. (Note that H+ is a catalyst in this reaction - it participates in the reaction but is not itself consumed)

-The rate constant for this reaction is:
A) smaller than 1 x 10-5
B) between 1 x 10-5 and 1x 10-4
C) between 1 x 10-4 and 1 x 10-3
D) between 1 x 10-3 and 1
E) greater than 1
The initial rate of disappearance of bromine (Br2) for the reaction shown below was measured for several different concentrations of bromine, CH3COCH3, and H+ ions. (Note that H+ is a catalyst in this reaction - it participates in the reaction but is not itself consumed)

-The rate constant for this reaction is:
A) smaller than 1 x 10-5
B) between 1 x 10-5 and 1x 10-4
C) between 1 x 10-4 and 1 x 10-3
D) between 1 x 10-3 and 1
E) greater than 1

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
28
The reaction shown below is first order in oxygen and second order in NO and has a rate constant of 7.1 x 109 M-2s-1. What would the reaction rate be when the NO concentration is 0.0010 M and the O2 concentration is 0.034 M?
2NO + O2 2NO2
A) 2.4 x 105 M/s
B) 3.4 x 10-8 M/s
C) 3.7 x 103 M/s
D) 8.9 x 10-5 M/s
E) 2.4 x 102 M/s
2NO + O2 2NO2
A) 2.4 x 105 M/s
B) 3.4 x 10-8 M/s
C) 3.7 x 103 M/s
D) 8.9 x 10-5 M/s
E) 2.4 x 102 M/s

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
29
The reaction following reaction was studied at 25  C, and the following experimental results were obtained.
C, and the following experimental results were obtained.
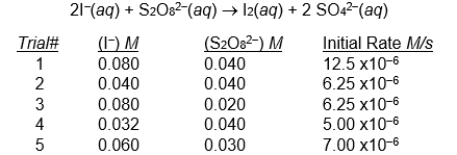
Which 2 trials of experimental data should be chosen to find the order of the reaction with respect to S2O82F1 - ?
A) trial 1 and 2
B) trial 2 and 3
C) trial 2 and 5
D) trial 1 and 3
E) none of these
 C, and the following experimental results were obtained.
C, and the following experimental results were obtained.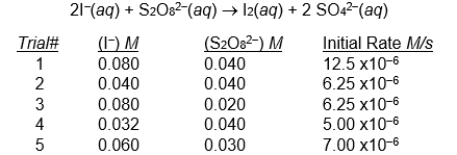
Which 2 trials of experimental data should be chosen to find the order of the reaction with respect to S2O82F1 - ?
A) trial 1 and 2
B) trial 2 and 3
C) trial 2 and 5
D) trial 1 and 3
E) none of these

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
30
Use the following data on the initial rate of reaction to determine the overall order of the reaction. 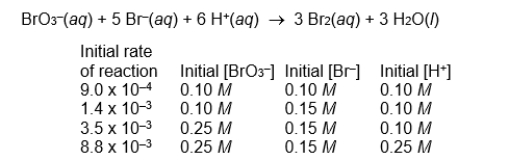
A) first-order overall
B) second-order overall
C) third-order overall
D) fourth-order overall
E) none of these

A) first-order overall
B) second-order overall
C) third-order overall
D) fourth-order overall
E) none of these

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
31
refer to the reaction for which the following data were obtained.
2 NO(g) + Br2(g) 2 NOBr(g)

-The rate law for this reaction would be:
A) zero-order in NO
B) half-order in NO
C) first-order in NO
D) second-order in NO
E) none of the above
2 NO(g) + Br2(g) 2 NOBr(g)

-The rate law for this reaction would be:
A) zero-order in NO
B) half-order in NO
C) first-order in NO
D) second-order in NO
E) none of the above

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
32
refer to the reaction for which the following data were obtained.
2 NO(g) + Br2(g) 2 NOBr(g)

-The rate law for this reaction would be:
A) zero-order in Br2
B) half-order in Br2
C) first-order in Br2
D) second-order in Br2
E) none of the above
2 NO(g) + Br2(g) 2 NOBr(g)

-The rate law for this reaction would be:
A) zero-order in Br2
B) half-order in Br2
C) first-order in Br2
D) second-order in Br2
E) none of the above

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
33
refer to the reaction for which the following data were obtained.
2 NO(g) + Br2(g) 2 NOBr(g)

-If these data were obtained by watching the rate at which NO disappears, what would be the initial instantaneous rate of disappearance of Br2 in Trial 1?
A) 0.30 M/s
B) 0.60 M/s
C) 1.2 M/s
D) 2.4 M/s
E) 4.8 M/s
2 NO(g) + Br2(g) 2 NOBr(g)

-If these data were obtained by watching the rate at which NO disappears, what would be the initial instantaneous rate of disappearance of Br2 in Trial 1?
A) 0.30 M/s
B) 0.60 M/s
C) 1.2 M/s
D) 2.4 M/s
E) 4.8 M/s

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
34
refer to the reaction for which the following data were obtained.
2 NO(g) + Br2(g) 2 NOBr(g)

-The magnitude of the rate constant, k, for this reaction is:
A) less than 1 x 10-4
B) between 10-4 and 10-2
C) between 0.01 and 100
D) between 100 and 10,000
E) more than 10,000
2 NO(g) + Br2(g) 2 NOBr(g)

-The magnitude of the rate constant, k, for this reaction is:
A) less than 1 x 10-4
B) between 10-4 and 10-2
C) between 0.01 and 100
D) between 100 and 10,000
E) more than 10,000

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
35
refer to the reaction for which the following data were obtained.
2 NO(g) + Br2(g) 2 NOBr(g)

-A plot of (NO) versus time would most closely resemble:
A) a straight line with a positive slope.
B) a straight line with a negative slope.
C) a straight line with a slope of zero.
D) a curve in which the NO concentration increases rapidly at first and then slows down until eventually a maximum concentration is achieved.
E) a curve in which the NO concentration decreases rapidly at first and then slows down until eventually a minimum concentration is achieved.
2 NO(g) + Br2(g) 2 NOBr(g)

-A plot of (NO) versus time would most closely resemble:
A) a straight line with a positive slope.
B) a straight line with a negative slope.
C) a straight line with a slope of zero.
D) a curve in which the NO concentration increases rapidly at first and then slows down until eventually a maximum concentration is achieved.
E) a curve in which the NO concentration decreases rapidly at first and then slows down until eventually a minimum concentration is achieved.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
36
refer to the reaction
Cr2O72-(aq) + 6 I-(aq) + 14 H+(aq) 2 Cr3+(aq) + 3 I2(aq) + 7 H2O(l)
for which the following initial instantaneous rates of reaction were obtained.
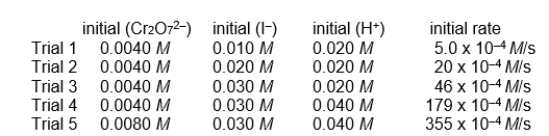
-If the initial rate data are for the rate at which I- disappears, -d(I-)/dt, what would be the rate of disappearance of Cr2O72- in Trial 1?
A) 0.83 x 10-4 M/s
B) 2.5 x 10-4 M/s
C) 5 x 10-4 M/s
D) 10 x 10-4 M/s
E) 30 x 10-4 M/s
Cr2O72-(aq) + 6 I-(aq) + 14 H+(aq) 2 Cr3+(aq) + 3 I2(aq) + 7 H2O(l)
for which the following initial instantaneous rates of reaction were obtained.

-If the initial rate data are for the rate at which I- disappears, -d(I-)/dt, what would be the rate of disappearance of Cr2O72- in Trial 1?
A) 0.83 x 10-4 M/s
B) 2.5 x 10-4 M/s
C) 5 x 10-4 M/s
D) 10 x 10-4 M/s
E) 30 x 10-4 M/s

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
37
refer to the reaction
Cr2O72-(aq) + 6 I-(aq) + 14 H+(aq) 2 Cr3+(aq) + 3 I2(aq) + 7 H2O(l)
for which the following initial instantaneous rates of reaction were obtained.

-The rate law for this reaction would be:
A) zero-order in Cr2O72-
B) half-order in Cr2O72-
C) first-order in Cr2O72-
D) second-order in Cr2O72-
E) none of the above
Cr2O72-(aq) + 6 I-(aq) + 14 H+(aq) 2 Cr3+(aq) + 3 I2(aq) + 7 H2O(l)
for which the following initial instantaneous rates of reaction were obtained.

-The rate law for this reaction would be:
A) zero-order in Cr2O72-
B) half-order in Cr2O72-
C) first-order in Cr2O72-
D) second-order in Cr2O72-
E) none of the above

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
38
refer to the reaction
Cr2O72-(aq) + 6 I-(aq) + 14 H+(aq) 2 Cr3+(aq) + 3 I2(aq) + 7 H2O(l)
for which the following initial instantaneous rates of reaction were obtained.

-The rate law for this reaction would be:
A) first-order in both I- and H+
B) first-order in I- and second-order in H+
C) first-order in H+ and second-order in I-
D) second-order in both H+ and I-
E) none of the above
Cr2O72-(aq) + 6 I-(aq) + 14 H+(aq) 2 Cr3+(aq) + 3 I2(aq) + 7 H2O(l)
for which the following initial instantaneous rates of reaction were obtained.

-The rate law for this reaction would be:
A) first-order in both I- and H+
B) first-order in I- and second-order in H+
C) first-order in H+ and second-order in I-
D) second-order in both H+ and I-
E) none of the above

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
39
refer to the reaction
Cr2O72-(aq) + 6 I-(aq) + 14 H+(aq) 2 Cr3+(aq) + 3 I2(aq) + 7 H2O(l)
for which the following initial instantaneous rates of reaction were obtained.

-The rate constant, k, for this reaction is:
A) less than 10-6
B) between 10-6 and 10-3
C) between 10-3 and 103
D) between 103 and 106
E) more than 106
Cr2O72-(aq) + 6 I-(aq) + 14 H+(aq) 2 Cr3+(aq) + 3 I2(aq) + 7 H2O(l)
for which the following initial instantaneous rates of reaction were obtained.

-The rate constant, k, for this reaction is:
A) less than 10-6
B) between 10-6 and 10-3
C) between 10-3 and 103
D) between 103 and 106
E) more than 106

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
40
refer to the reaction
Cr2O72-(aq) + 6 I-(aq) + 14 H+(aq) 2 Cr3+(aq) + 3 I2(aq) + 7 H2O(l)
for which the following initial instantaneous rates of reaction were obtained.

-A graph of (Cr2O72-) versus time would most closely resemble
A) a straight line with a negative slope.
B) a straight line with a slope of zero.
C) a straight line with a positive slope.
D) a curve in which the Cr2O72- concentration decreases rapidly at first, and then the rate at which Cr2O72- disappears slows down with time.
E) a curve in which the Cr2O72- concentration increases rapidly at first, and then the rate at which Cr2O72- appears slows down with time.
Cr2O72-(aq) + 6 I-(aq) + 14 H+(aq) 2 Cr3+(aq) + 3 I2(aq) + 7 H2O(l)
for which the following initial instantaneous rates of reaction were obtained.

-A graph of (Cr2O72-) versus time would most closely resemble
A) a straight line with a negative slope.
B) a straight line with a slope of zero.
C) a straight line with a positive slope.
D) a curve in which the Cr2O72- concentration decreases rapidly at first, and then the rate at which Cr2O72- disappears slows down with time.
E) a curve in which the Cr2O72- concentration increases rapidly at first, and then the rate at which Cr2O72- appears slows down with time.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
41
A certain substance, initially at 0.10 M in solution, decomposes by second-order kinetics. If the rate constant for this process is 0.40 M - 1 min - 1, how much time in minutes is required for the concentration to reach 0.020 M?
A) 1.6 x 10 - 4 min
B) 8.0 x 10 - 3 min
C) 4.02 min
D) 50 min
E) 100 min
A) 1.6 x 10 - 4 min
B) 8.0 x 10 - 3 min
C) 4.02 min
D) 50 min
E) 100 min

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
42
The reaction A C is found to be zero order. Which of the following will give a linear plot?
A) (A) vs. time
B) ln(A) vs. time
C) 1/(A) vs. time
D) None of these will be linear.
A) (A) vs. time
B) ln(A) vs. time
C) 1/(A) vs. time
D) None of these will be linear.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
43
The half-life for the first-order decomposition of nitramide, NH2NO2, into nitrous oxide, N2O, and water is 123 min at 15°C.
NH2NO2 N2O + H2O
What is the value of the rate constant (k) for this reaction?
NH2NO2 N2O + H2O
What is the value of the rate constant (k) for this reaction?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
44
For the reaction in the above question, how long will it take in minutes for 2.0 g of nitramide to decompose until only 0.20 g remains?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following graphs describes the relationship between rate and time for a first-order reaction?
A)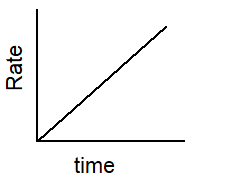
B)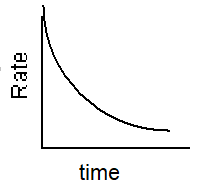
C)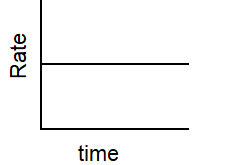
D)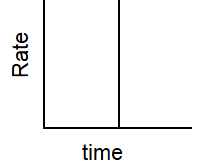
E)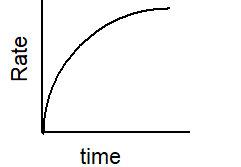
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
46
Which plot best describe the rate of a second-order reaction?
2 A B
A)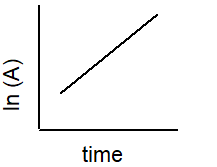
B)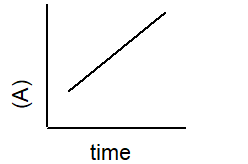
C)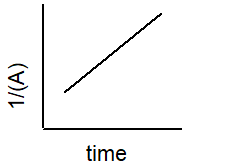
D)
E)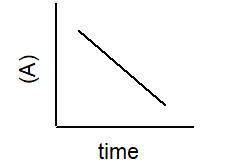
2 A B
A)

B)

C)

D)
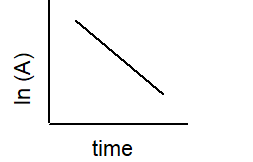
E)


Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
47
A certain second-order reaction is found to have a rate constant of
0.135 M-1 s-1 What is the half-life of the reaction?
A) 5.1 s
B) 6.5 s
C) 7.4 s
D) none of these
E) impossible to determine from this information
0.135 M-1 s-1 What is the half-life of the reaction?
A) 5.1 s
B) 6.5 s
C) 7.4 s
D) none of these
E) impossible to determine from this information

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following statements about the half-life of a reaction is true?
A) The half-life doesn't depend on the order of the reaction.
B) The half-life of a first-order reaction increases with time.
C) The half-life of a second-order reaction is independent of concentration.
D) A zero-order reaction doesn't have a half-life.
E) None of the above are true.
A) The half-life doesn't depend on the order of the reaction.
B) The half-life of a first-order reaction increases with time.
C) The half-life of a second-order reaction is independent of concentration.
D) A zero-order reaction doesn't have a half-life.
E) None of the above are true.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
49
For a second-order reaction, it takes 15 seconds for the initial concentration of a reactant to decrease from 0.60 M to 0.53 M. What is the initial half-life of this reaction?
A) 47 s
B) 94 s
C) 114 s
D) 128 s
E) 228 s
A) 47 s
B) 94 s
C) 114 s
D) 128 s
E) 228 s

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
50
The rate constant, k, for the first-order decay of 14C is 1.21 x 10-4 yr. If analysis of a piece of paper, parchment, or papyri suggests that 97.4% of the 14C that was present initially still remains in the sample, it would most likely be associated with which of the following battles?
A) Battle of Actium, 31 BC, Octavian defeating Mark Anthony
B) Battle of Hastings, 1066 AD, William of Normandy defeating King Harold II of England
C) Battle of Stirling Bridge, 1297 AD, William Wallace defeating John de Warrenne, Earl of Surrey
D) Battle of Yorktown, 1781 AD, Washington defeating Lord Cornwallis
E) Battle of Waterloo, 1815 AD, Wellington defeating Napoleon
A) Battle of Actium, 31 BC, Octavian defeating Mark Anthony
B) Battle of Hastings, 1066 AD, William of Normandy defeating King Harold II of England
C) Battle of Stirling Bridge, 1297 AD, William Wallace defeating John de Warrenne, Earl of Surrey
D) Battle of Yorktown, 1781 AD, Washington defeating Lord Cornwallis
E) Battle of Waterloo, 1815 AD, Wellington defeating Napoleon

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
51
The decay of radioactive nuclei is a first-order kinetic process. Determine the half-life for the decay of 238U to 206Pb if the first-order rate constant is 4.87 x 10-18 s-1
A) 3.37 x 10-18 s
B) 39.5 s
C) 1.42 x 1017 s
D) 4.11 x 1017 s
E) none of these
A) 3.37 x 10-18 s
B) 39.5 s
C) 1.42 x 1017 s
D) 4.11 x 1017 s
E) none of these

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
52
The half-life of 228Ra is 1590 years. How long will it take for a sample of this nuclide to decay to 1.0% of its original activity?
A) 16 years
B) 1.6 x 103 years
C) 4.1 x 103 years
D) 1.1 x 104 years
E) none of these
A) 16 years
B) 1.6 x 103 years
C) 4.1 x 103 years
D) 1.1 x 104 years
E) none of these

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
53
The rate of a zero-order reaction
A) increases as reactant is consumed.
B) depends on the concentration of products.
C) decreases as reactant is consumed.
D) is independent of temperature.
E) is independent of the concentration of reactants and products.
A) increases as reactant is consumed.
B) depends on the concentration of products.
C) decreases as reactant is consumed.
D) is independent of temperature.
E) is independent of the concentration of reactants and products.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
54
The reaction, N2O5 NO2 + 1/2 O2, is first-order in N2O5 with a half-life of 19.25 min. How long would it take in minutes for the N2O5 concentration to decrease from 0.050 M to 0.030 M?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
55
The following data table should be used to answer
Reaction: A B
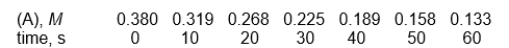
-What is the rate constant, k, for this reaction?
A) 0.0175
B) 0.0560
C) 0.0815
D) 0.112
E) none of these
Reaction: A B
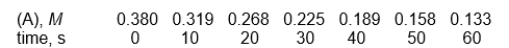
-What is the rate constant, k, for this reaction?
A) 0.0175
B) 0.0560
C) 0.0815
D) 0.112
E) none of these

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
56
The following data table should be used to answer
Reaction: A B
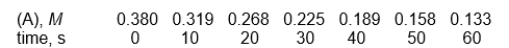
-What is the half-life for this reaction, in seconds?
A) 29.1
B) 39.6
C) 47.3
D) 151
E) none of these
Reaction: A B
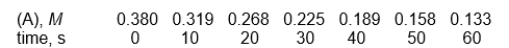
-What is the half-life for this reaction, in seconds?
A) 29.1
B) 39.6
C) 47.3
D) 151
E) none of these

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
57
The following data table should be used to answer
Reaction: A B
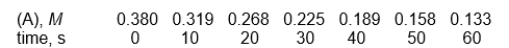
-What will be the concentration of A when t = 120 seconds?
A) 0.047
B) 0.10
C) 0.13
D) 0.21
E) none of these
Reaction: A B
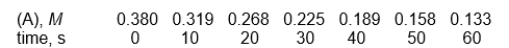
-What will be the concentration of A when t = 120 seconds?
A) 0.047
B) 0.10
C) 0.13
D) 0.21
E) none of these

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
58
If a plot of 1/[A] versus time produces a straight line, which of the following is true?
A) This reaction is first-order in A.
B) The reaction is second-order in A.
C) The reaction is first-order in two reactants.
D) The rate of reaction does not depend on the concentration of A.
E) none of the above
A) This reaction is first-order in A.
B) The reaction is second-order in A.
C) The reaction is first-order in two reactants.
D) The rate of reaction does not depend on the concentration of A.
E) none of the above

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
59
Use the following data to answer
These data were obtained by monitoring the rate at which the OCl- ion was consumed in the presence of a large excess of the I- ion.
OCl-(aq) + I-(aq) OI-(aq) + Cl-(aq)
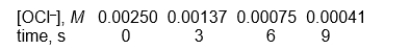
-What is the rate constant (k) for this reaction?
A) between 10-2 and 10-1
B) larger than 10-1 and smaller than 1
C) between 1 and 10
D) larger than 10 and smaller than 100
E) between 100 and 1000
These data were obtained by monitoring the rate at which the OCl- ion was consumed in the presence of a large excess of the I- ion.
OCl-(aq) + I-(aq) OI-(aq) + Cl-(aq)
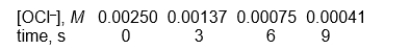
-What is the rate constant (k) for this reaction?
A) between 10-2 and 10-1
B) larger than 10-1 and smaller than 1
C) between 1 and 10
D) larger than 10 and smaller than 100
E) between 100 and 1000

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
60
Use the following data to answer
These data were obtained by monitoring the rate at which the OCl- ion was consumed in the presence of a large excess of the I- ion.
OCl-(aq) + I-(aq) OI-(aq) + Cl-(aq)
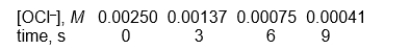
-What is the half-life in seconds?
A) between 0.01 and 0.1 seconds
B) between 0.1 and 1 second
C) between 1 and 10 seconds
D) between 10 and 100 seconds
E) none of the above
These data were obtained by monitoring the rate at which the OCl- ion was consumed in the presence of a large excess of the I- ion.
OCl-(aq) + I-(aq) OI-(aq) + Cl-(aq)
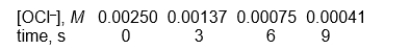
-What is the half-life in seconds?
A) between 0.01 and 0.1 seconds
B) between 0.1 and 1 second
C) between 1 and 10 seconds
D) between 10 and 100 seconds
E) none of the above

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
61
Use the following data to answer
These data were obtained by monitoring the rate at which the OCl- ion was consumed in the presence of a large excess of the I- ion.
OCl-(aq) + I-(aq) OI-(aq) + Cl-(aq)
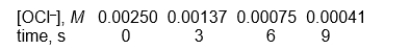
-What is the concentration of OCl- after 12 seconds?
A) between 1 x 10-4 and 2 x 10-4
B) between 2 x 10-4 and 3 x 10-4
C) between 3 x 10-4 and 4 x 10-4
D) between 4 x 10-4 and 5 x 10-4
E) between 5 x 10-4 and 6 x 10-4
These data were obtained by monitoring the rate at which the OCl- ion was consumed in the presence of a large excess of the I- ion.
OCl-(aq) + I-(aq) OI-(aq) + Cl-(aq)
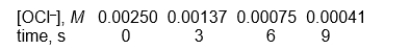
-What is the concentration of OCl- after 12 seconds?
A) between 1 x 10-4 and 2 x 10-4
B) between 2 x 10-4 and 3 x 10-4
C) between 3 x 10-4 and 4 x 10-4
D) between 4 x 10-4 and 5 x 10-4
E) between 5 x 10-4 and 6 x 10-4

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
62
refer to the following reaction for the decomposition of H2O2.
2 H2O2(aq) 2 H2O(l) + O2(g)
The following data were obtained
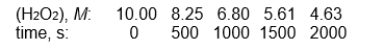
-The rate law for this reaction is:
A) zero-order in H2O2
B) first-order in H2O2
C) second-order in H2O2
D) third-order in H2O2
E) none of these
2 H2O2(aq) 2 H2O(l) + O2(g)
The following data were obtained
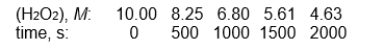
-The rate law for this reaction is:
A) zero-order in H2O2
B) first-order in H2O2
C) second-order in H2O2
D) third-order in H2O2
E) none of these

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
63
refer to the following reaction for the decomposition of H2O2.
2 H2O2(aq) 2 H2O(l) + O2(g)
The following data were obtained
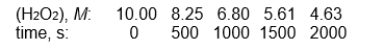
-The half-life for this reaction is:
A) between 0 and 50 s
B) between 50 and 500 s
C) between 500 and 1500 s
D) between 1500 and 2000 s
E) the half-life can't be obtained from these data
2 H2O2(aq) 2 H2O(l) + O2(g)
The following data were obtained
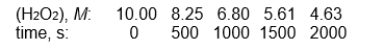
-The half-life for this reaction is:
A) between 0 and 50 s
B) between 50 and 500 s
C) between 500 and 1500 s
D) between 1500 and 2000 s
E) the half-life can't be obtained from these data

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
64
refer to the following reaction for the decomposition of H2O2.
2 H2O2(aq) 2 H2O(l) + O2(g)
The following data were obtained
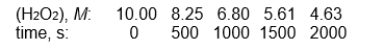
-What will be the H2O2 concentration after 4000 seconds?
A) less than 1.5 M
B) between 1.5 and 2.5 M
C) between 2.5 and 3.0 M
D) between 3.0 and 3.5 M
E) between 3.5 and 4.5 M
2 H2O2(aq) 2 H2O(l) + O2(g)
The following data were obtained
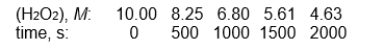
-What will be the H2O2 concentration after 4000 seconds?
A) less than 1.5 M
B) between 1.5 and 2.5 M
C) between 2.5 and 3.0 M
D) between 3.0 and 3.5 M
E) between 3.5 and 4.5 M

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
65
refer to the reaction
2 N2O5(g) 4 NO2(g) + O2(g)
for which the following data were obtained.

-The rate law for this reaction would be:
A) zero-order in N2O5
B) half-order in N2O5
C) first-order in N2O5
D) second-order in N2O5
E) none of the above
2 N2O5(g) 4 NO2(g) + O2(g)
for which the following data were obtained.

-The rate law for this reaction would be:
A) zero-order in N2O5
B) half-order in N2O5
C) first-order in N2O5
D) second-order in N2O5
E) none of the above

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
66
refer to the reaction
2 N2O5(g) 4 NO2(g) + O2(g)
for which the following data were obtained.

-The half-life for this reaction is:
A) between 0 and 12 s
B) between 12 and 120 s
C) between 120 and 1200 s
D) between 1200 and 12,000 s
E) between 12,000 and 120,000 s
2 N2O5(g) 4 NO2(g) + O2(g)
for which the following data were obtained.

-The half-life for this reaction is:
A) between 0 and 12 s
B) between 12 and 120 s
C) between 120 and 1200 s
D) between 1200 and 12,000 s
E) between 12,000 and 120,000 s

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
67
refer to the reaction
2 N2O5(g) 4 NO2(g) + O2(g)
for which the following data were obtained.

-What will be the concentration of N2O5 after 5000 seconds?
A) between 0.001 and 0.010 M
B) between 0.010 and 0.10 M
C) between 0.10 and 0.4 M
D) between 0.4 and 0.8 M
E) between 0.8 and 1.2 M
2 N2O5(g) 4 NO2(g) + O2(g)
for which the following data were obtained.

-What will be the concentration of N2O5 after 5000 seconds?
A) between 0.001 and 0.010 M
B) between 0.010 and 0.10 M
C) between 0.10 and 0.4 M
D) between 0.4 and 0.8 M
E) between 0.8 and 1.2 M

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
68
refer to the reaction
2 H2O2(aq)
 2 H2O(l) + O2(g)
2 H2O(l) + O2(g)
for which the following data were obtained at 25%C.

-The rate law for this reaction is
A) zero-order in hydrogen peroxide
B) half-order in hydrogen peroxide
C) first-order in hydrogen peroxide
D) second-order in hydrogen peroxide
E) none of the above
2 H2O2(aq)
 2 H2O(l) + O2(g)
2 H2O(l) + O2(g)for which the following data were obtained at 25%C.

-The rate law for this reaction is
A) zero-order in hydrogen peroxide
B) half-order in hydrogen peroxide
C) first-order in hydrogen peroxide
D) second-order in hydrogen peroxide
E) none of the above

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
69
refer to the reaction
2 H2O2(aq)
 2 H2O(l) + O2(g)
2 H2O(l) + O2(g)
for which the following data were obtained at 25%C.

-When will the concentration of H2O2 reach 0.010 M?
A) before 200 s
B) between 200 and 240 s
C) between 240 and 280 s
D) between 280 and 320 s
E) after 320 s
2 H2O2(aq)
 2 H2O(l) + O2(g)
2 H2O(l) + O2(g)for which the following data were obtained at 25%C.

-When will the concentration of H2O2 reach 0.010 M?
A) before 200 s
B) between 200 and 240 s
C) between 240 and 280 s
D) between 280 and 320 s
E) after 320 s

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
70
H2 and O2 can react explosively. However, a mixture of H2 and O2 can exist indefinitely at room temperature with no reaction occurring. Explain why hydrogen and oxygen do not react under these conditions.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
71
If the rate constant increases from 0.40 M-1 s-1 at 25  C to 0.80 M-1 s-1 at 35 11ee9f13_6ea8_431e_9fac_3dff7a14c0f9_TB9692_11 C, what is the activation energy in kJ/mol for this reaction?
C to 0.80 M-1 s-1 at 35 11ee9f13_6ea8_431e_9fac_3dff7a14c0f9_TB9692_11 C, what is the activation energy in kJ/mol for this reaction?
A) between 0 and 40 kJ mol
B) between 41 and 80 kJ/mol
C) between 81 and 120 kJ/mol
D) between 121 and 160 kJ/mol
E) between 161 and 200 kJ/mol
 C to 0.80 M-1 s-1 at 35 11ee9f13_6ea8_431e_9fac_3dff7a14c0f9_TB9692_11 C, what is the activation energy in kJ/mol for this reaction?
C to 0.80 M-1 s-1 at 35 11ee9f13_6ea8_431e_9fac_3dff7a14c0f9_TB9692_11 C, what is the activation energy in kJ/mol for this reaction?A) between 0 and 40 kJ mol
B) between 41 and 80 kJ/mol
C) between 81 and 120 kJ/mol
D) between 121 and 160 kJ/mol
E) between 161 and 200 kJ/mol

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
72
A chemical reaction is found to be endothermic. Which of the following correctly describes the activation energy associated with the forward and the reverse reactions?
A) Ea forward = Ea reverse
B) Ea forward < Ea reverse at all temperatures
C) Ea forward > Ea reverse at all temperatures
D) Ea forward = Ea reverse at low temperatures
E) none of these
A) Ea forward = Ea reverse
B) Ea forward < Ea reverse at all temperatures
C) Ea forward > Ea reverse at all temperatures
D) Ea forward = Ea reverse at low temperatures
E) none of these

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
73
The activation energy was measured for both the forward (Ea=150 kJ/molrxn) and reverse (Ea = 95 kJ/molrxn) directions of a reversible reaction. What would be the activation energy for the reverse reaction in the presence of a catalyst that decreased the activation energy for the forward reaction to
125 kJ/ molrxn?
A) 25 kJ/molrxn
B) 75 kJ/ molrxn
C) 120 kJ/ molrxn
D) 30 kJ/ molrxn
E) none of these
125 kJ/ molrxn?
A) 25 kJ/molrxn
B) 75 kJ/ molrxn
C) 120 kJ/ molrxn
D) 30 kJ/ molrxn
E) none of these

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following correctly describes the variation of rate constant, kf, with temperature?
A) kf = ln Z - Ea/RT
B) ln kf = ln Z + e-(Ea/RT)
C) ln kf = ln Z - Ea/RT
D) ln kf = ln Z + Ea/RT.
E) kf = ln Z - Ea/RT.
A) kf = ln Z - Ea/RT
B) ln kf = ln Z + e-(Ea/RT)
C) ln kf = ln Z - Ea/RT
D) ln kf = ln Z + Ea/RT.
E) kf = ln Z - Ea/RT.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
75
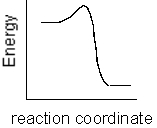
Which of the following plots represents the largest rate constant for an endothermic reaction?
A)
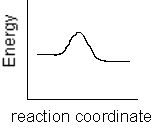
B)
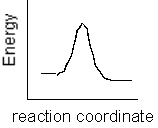
C)
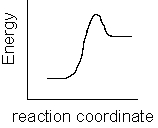
D)
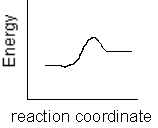
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
76
For the gas phase decomposition of N2O2, k is 1.4 s - 1 at 400 K and 43 s - 1 at 450 K. What is the activation energy for this reaction?
A) 102 kJ/molrxn
B) 3.42 kJ/molrxn
C) 50.0 kJ/molrxn
D) 232 kJ/molrxn
E) 78.4 kJ/molrxn
A) 102 kJ/molrxn
B) 3.42 kJ/molrxn
C) 50.0 kJ/molrxn
D) 232 kJ/molrxn
E) 78.4 kJ/molrxn

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
77
Which statement is true?
A) The rate of appearance of products of a chemical reaction is always equal to the rate of disappearance of reactants.
B) If a reaction follows a second-order rate law, it must have two steps in its reaction mechanism.
C) The half-life for a first-order reaction is always larger than the half-life for a second-order reaction.
D) The half-life for a first-order reaction is independent of the initial concentration of the reactant.
E) The half-life for a second-order reaction is independent of the initial concentration of the reactant.
A) The rate of appearance of products of a chemical reaction is always equal to the rate of disappearance of reactants.
B) If a reaction follows a second-order rate law, it must have two steps in its reaction mechanism.
C) The half-life for a first-order reaction is always larger than the half-life for a second-order reaction.
D) The half-life for a first-order reaction is independent of the initial concentration of the reactant.
E) The half-life for a second-order reaction is independent of the initial concentration of the reactant.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
78
For the reaction, 2 NOCl(g) 2 NO(g) + Cl2(g),
The observed rate expression is rate = k(NOCl)2. Which of the conditions listed below would cause a change in the value of the rate constant, k?
A) increase the concentration of NOCl
B) decrease the concentration of NOCl
C) decrease the concentration of NO
D) increase the temperature
E) increase the concentration of NOCl and decrease the concentration of NO and Cl2
The observed rate expression is rate = k(NOCl)2. Which of the conditions listed below would cause a change in the value of the rate constant, k?
A) increase the concentration of NOCl
B) decrease the concentration of NOCl
C) decrease the concentration of NO
D) increase the temperature
E) increase the concentration of NOCl and decrease the concentration of NO and Cl2

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
79
As the reaction below proceeds, what happens (increase, decrease, or remain constant) to the rate of the forward reaction?
CO(g) + H2O(g) CO2(g) + H2(g)
As the reaction proceeds what happens to the rate of the reverse reaction (CO2 + H2 CO + H2O)?
Explain the above answers using the collision theory for gas-phase reactions.
CO(g) + H2O(g) CO2(g) + H2(g)
As the reaction proceeds what happens to the rate of the reverse reaction (CO2 + H2 CO + H2O)?
Explain the above answers using the collision theory for gas-phase reactions.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck


