Deck 5: Atoms, Molecules, Formulas, and Subatomic Particles
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/87
Play
Full screen (f)
Deck 5: Atoms, Molecules, Formulas, and Subatomic Particles
1
Which of the following statements is not a part of atomic theory?
A) Chemical change involves a union, separation, or rearrangement of atoms.
B) A pure substance will have a varying relative number of atom types.
C) All matter is made up of atoms.
D) Atoms are considered indestructible during chemical change.
A) Chemical change involves a union, separation, or rearrangement of atoms.
B) A pure substance will have a varying relative number of atom types.
C) All matter is made up of atoms.
D) Atoms are considered indestructible during chemical change.
A pure substance will have a varying relative number of atom types.
2
The diameters and masses of atoms are, respectively, on the order of ________.
A) 10-11 m and 10-11 g
B) 10-23 m and 10-10 g
C) 10-24 m and 10-10 g
D) 10-10 m and 10-23 g
A) 10-11 m and 10-11 g
B) 10-23 m and 10-10 g
C) 10-24 m and 10-10 g
D) 10-10 m and 10-23 g
10-10 m and 10-23 g
3
Which one of the following statements about atoms is correct?
A) The proton is the smallest "piece" of an atom that can exist and still have the properties of the atom.
B) Free, isolated atoms are virtually never encountered in nature.
C) Atoms may be decomposed using physical change.
D) Two kinds of atoms may be present in a homoatomic molecule.
A) The proton is the smallest "piece" of an atom that can exist and still have the properties of the atom.
B) Free, isolated atoms are virtually never encountered in nature.
C) Atoms may be decomposed using physical change.
D) Two kinds of atoms may be present in a homoatomic molecule.
Free, isolated atoms are virtually never encountered in nature.
4
Which one of the following statements about molecules is correct?
A) Some compounds have molecules as their basic structural unit.
B) For a molecular compound, the atom is the limit of chemical subdivision.
C) For a molecular compound, the atom is the limit of physical subdivision.
D) Molecules of compounds may be either heteroatomic or homoatomic.
A) Some compounds have molecules as their basic structural unit.
B) For a molecular compound, the atom is the limit of chemical subdivision.
C) For a molecular compound, the atom is the limit of physical subdivision.
D) Molecules of compounds may be either heteroatomic or homoatomic.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
5
Which one of the following statements about heteroatomic molecules is incorrect?
A) Atoms in a heteroatomic molecule may be the same atom.
B) Upon chemical subdivision, heteroatomic molecules always yield two or more kinds of atoms.
C) Heteroatomic molecules do not maintain the properties of their constituent elements.
D) Elements are never heteroatomic molecules.
A) Atoms in a heteroatomic molecule may be the same atom.
B) Upon chemical subdivision, heteroatomic molecules always yield two or more kinds of atoms.
C) Heteroatomic molecules do not maintain the properties of their constituent elements.
D) Elements are never heteroatomic molecules.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
6
Which statement about ionic compounds is incorrect?
A) Ionic compounds do not contain individual molecules.
B) Ionic compounds are charged, they are not electrically neutral.
C) Ionic compounds have an extended 3-dimensional assembly of ions.
D) Ionic compounds do not share the characteristics of the constituent elements.
A) Ionic compounds do not contain individual molecules.
B) Ionic compounds are charged, they are not electrically neutral.
C) Ionic compounds have an extended 3-dimensional assembly of ions.
D) Ionic compounds do not share the characteristics of the constituent elements.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
7
Which statement concerning the molecules depicted is incorrect?
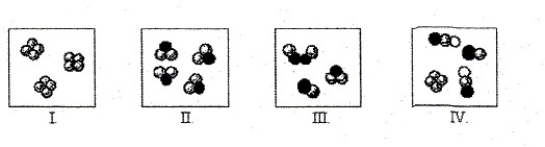
A) The molecules in box I represent a pure substance.
B) The molecules in box III represent a mixture of elements.
C) The molecules in box II represent a compound composed of two different elements.
D) The molecules in box IV represents two different compounds and an element.

A) The molecules in box I represent a pure substance.
B) The molecules in box III represent a mixture of elements.
C) The molecules in box II represent a compound composed of two different elements.
D) The molecules in box IV represents two different compounds and an element.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
8
In the formula C4H8O2 ________.
A) there are 2 different elements
B) the ratio of carbon to hydrogen to oxygen atoms is 2:4:1
C) the percentage by mass of hydrogen is the same as oxygen
D) there is a total of 10 atoms
A) there are 2 different elements
B) the ratio of carbon to hydrogen to oxygen atoms is 2:4:1
C) the percentage by mass of hydrogen is the same as oxygen
D) there is a total of 10 atoms

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
9
In which of the following compounds does one molecule of the compound contain 3 elements and 8 atoms?
A) NaHCO3
B) C2H7N
C) H3AsO4
D) POCl3
A) NaHCO3
B) C2H7N
C) H3AsO4
D) POCl3

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
10
In which of the following pairs of formulas do the two members of the pair contain the same number of elements as well as the same number of atoms?
A) Co and CO
B) NaHCO3 and NaHSO4
C) SO3 and SO2
D) Cl2CO and C2HCl
A) Co and CO
B) NaHCO3 and NaHSO4
C) SO3 and SO2
D) Cl2CO and C2HCl

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
11
The total number of atoms present in 2 formula units of Ca(NO3)2 is ________.
A) 9
B) 18
C) 25
D) 30
A) 9
B) 18
C) 25
D) 30

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
12
The total number of atoms present in five formula units of Sr(C2H3O2)2 is ________.
A) 65
B) 75
C) 18
D) 15
A) 65
B) 75
C) 18
D) 15

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
13
How many atoms of each element are present in 3 formula units of (NH4)2HAsO4?
A) 10 N atoms, 18 H atoms, 5 As atoms, 18 O atoms
B) 6 N atoms, 9 H atoms, 3 As atoms, 12 O atoms
C) 5 N atoms, 45 H atoms, 4 As atoms, 18 O atoms
D) 6 N atoms, 27 H atoms, 3 As atoms, 12 O atoms
A) 10 N atoms, 18 H atoms, 5 As atoms, 18 O atoms
B) 6 N atoms, 9 H atoms, 3 As atoms, 12 O atoms
C) 5 N atoms, 45 H atoms, 4 As atoms, 18 O atoms
D) 6 N atoms, 27 H atoms, 3 As atoms, 12 O atoms

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
14
Which formula is correct for a molecule with 4 P atoms, and 10 O atoms?
A) 3 P4O10
B) 2(P2O3)2
C) P4O10
D) 3 P3O6
A) 3 P4O10
B) 2(P2O3)2
C) P4O10
D) 3 P3O6

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
15
What is the total number of hydrogen atoms present in 6 formula units of Mg(C2H3O2)2?
A) 12
B) 6
C) 36
D) 24
A) 12
B) 6
C) 36
D) 24

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
16
What is the total number of atoms in 5 formula units of Fe2(C2O4)3?
A) 55
B) 80
C) 100
D) 96
A) 55
B) 80
C) 100
D) 96

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
17
What is the total number of O atoms in 6 formula units of hydroxyapetite, Ca10(PO4)6(OH)2, present in tooth enamel?
A) 156
B) 144
C) 106
D) 88
A) 156
B) 144
C) 106
D) 88

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following pairs of subatomic particles have charges of equal magnitude but opposite in sign?
A) electron and neutron
B) neutron and proton
C) proton and electron
D) proton and positron
A) electron and neutron
B) neutron and proton
C) proton and electron
D) proton and positron

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
19
The nucleus of an atom ________.
A) contains all protons and electrons
B) is negatively charged because of the presence of electrons
C) is neutral since it contains only neutrons
D) is 1/10,000 of the size of the atom
A) contains all protons and electrons
B) is negatively charged because of the presence of electrons
C) is neutral since it contains only neutrons
D) is 1/10,000 of the size of the atom

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
20
Atoms are neutral because ________.
A) all atoms contain neutrons
B) neutrons neutralize the (+) and (-) charges in the atom
C) the number of protons equals the number of electrons
D) equal numbers of protons and neutrons are present
A) all atoms contain neutrons
B) neutrons neutralize the (+) and (-) charges in the atom
C) the number of protons equals the number of electrons
D) equal numbers of protons and neutrons are present

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
21
Which statement about an atom is false?
A) The nucleus is the center region of an atom.
B) The space occupied by the electrons is primarily empty.
C) The nucleus accounts for a small portion of the mass of an atom.
D) The volume occupied by electrons is referred to as an electron cloud.
A) The nucleus is the center region of an atom.
B) The space occupied by the electrons is primarily empty.
C) The nucleus accounts for a small portion of the mass of an atom.
D) The volume occupied by electrons is referred to as an electron cloud.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
22
An atom contains 12 neutrons and 14 electrons. The mass number and the number of protons for this atom are, respectively, ________.
A) 14 and 26
B) 40 and 14
C) 26 and 14
D) 26 and 12
A) 14 and 26
B) 40 and 14
C) 26 and 14
D) 26 and 12

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
23
All atoms of a given element may have different numbers of ________.
A) gamma rays
B) nucleons
C) neutrons
D) protons
A) gamma rays
B) nucleons
C) neutrons
D) protons

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
24
What are the number of protons, neutrons, and electrons in 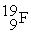 ?
?
A) 9 protons, 9 neutrons, and 10 electrons
B) 9 protons, 10 neutrons, and 9 electrons
C) 19 protons, 19 neutrons, and 19 electrons
D) 9 protons, 9 neutrons, and 19 electrons
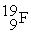 ?
?A) 9 protons, 9 neutrons, and 10 electrons
B) 9 protons, 10 neutrons, and 9 electrons
C) 19 protons, 19 neutrons, and 19 electrons
D) 9 protons, 9 neutrons, and 19 electrons

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
25
The number of electrons and neutrons in an atom of the type given below is ________. 25Mg
A) 12 e, 13 n
B) 25 e, 12 n
C) 13 e, 25 n
D) 12 e, 12 n
A) 12 e, 13 n
B) 25 e, 12 n
C) 13 e, 25 n
D) 12 e, 12 n

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
26
The number of neutrons in an atom is equal to:
A) the number of protons
B) atomic number - mass number
C) mass number - atomic number
D) the number of electrons
A) the number of protons
B) atomic number - mass number
C) mass number - atomic number
D) the number of electrons

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following statements is incorrect?
A) All atoms of an element have the same atomic number.
B) All atoms of an element must have the same mass.
C) All atoms of an element have the same number of electrons.
D) Atoms of an element may have different numbers of neutrons.
A) All atoms of an element have the same atomic number.
B) All atoms of an element must have the same mass.
C) All atoms of an element have the same number of electrons.
D) Atoms of an element may have different numbers of neutrons.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
28
The total number of subatomic particles (protons, electrons, and neutrons) in an atom of 75Ge is ________.
A) 86
B) 84
C) 107
D) 143
A) 86
B) 84
C) 107
D) 143

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
29
The number of protons, electrons and neutrons in an atom of the type given below is ________.
207Pb
A) 82 p, 125 e, 82 n
B) 82 p, 82 e, 125 n
C) 82 p, 125 e, 125 n
D) 125 p, 125 e, 164 n
207Pb
A) 82 p, 125 e, 82 n
B) 82 p, 82 e, 125 n
C) 82 p, 125 e, 125 n
D) 125 p, 125 e, 164 n

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
30
Choose the correct chemical symbol for an atom that contains 20 e? and 24 n.
A)
B)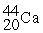
C)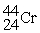
D)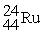
A)
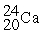
B)
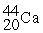
C)
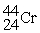
D)
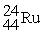

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
31
Isotopes of a given element ________.
A) have the same mass number but different numbers of protons
B) have the same number of protons, but different mass numbers
C) have the same atomic number but different chemical properties
D) have the same mass number but different chemical properties
A) have the same mass number but different numbers of protons
B) have the same number of protons, but different mass numbers
C) have the same atomic number but different chemical properties
D) have the same mass number but different chemical properties

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
32
The nucleus of atom A contains 20 protons and 20 neutrons and that of atom B contains 22 protons and 22 neutrons. It is true that atoms A and B are ________.
A) isotopes
B) isobars
C) both isotopes and isobars
D) neither isotopes nor isobars
A) isotopes
B) isobars
C) both isotopes and isobars
D) neither isotopes nor isobars

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
33
What is the mass number of a chromium isotope containing 28 neutrons?
A) 28
B) 24
C) 52
D) 76
A) 28
B) 24
C) 52
D) 76

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
34
Iron is element number 26. This means that it has ________.
A) a mass number of 26
B) 26 neutrons in the nucleus
C) 26 protons inside its nucleus
D) 56 electrons
A) a mass number of 26
B) 26 neutrons in the nucleus
C) 26 protons inside its nucleus
D) 56 electrons

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
35
What is the atomic number of an isobar of
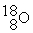 In which there is an equal number of all three types of subatomic particles present?
In which there is an equal number of all three types of subatomic particles present?
A) 8
B) 9
C) 10
D) 11
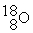 In which there is an equal number of all three types of subatomic particles present?
In which there is an equal number of all three types of subatomic particles present?A) 8
B) 9
C) 10
D) 11

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
36
Which pair of neutrons and protons correspond to the isobars of Ar and Ca, which have a mass number of 40?
A) Ar: 18 protons and 22 neutronsCa: 20 protons and 20 neutrons
B) Ar: 18 protons and 22 neutronsCa: 20 protons and 22 neutrons
C) Ar: 20 protons and 20 neutronsCa: 18 protons and 22 neutrons
D) Ar: 22 protons and 18 neutronsCa: 20 protons and 20 neutrons
A) Ar: 18 protons and 22 neutronsCa: 20 protons and 20 neutrons
B) Ar: 18 protons and 22 neutronsCa: 20 protons and 22 neutrons
C) Ar: 20 protons and 20 neutronsCa: 18 protons and 22 neutrons
D) Ar: 22 protons and 18 neutronsCa: 20 protons and 20 neutrons

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following pairs of atoms contain the same number of neutrons?
A) 27Al and 31P
B) 9Be and 7Li
C) 22Ne and 23Na
D) 58Ni and 58Fe
A) 27Al and 31P
B) 9Be and 7Li
C) 22Ne and 23Na
D) 58Ni and 58Fe

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following represents a pair of isotopes?
A) 14C, 14N
B) 1H, 2H
C) 32S, 32S-2
D) O2, O3
A) 14C, 14N
B) 1H, 2H
C) 32S, 32S-2
D) O2, O3

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following properties for the most abundant isotope of an element would not be possessed by the second most abundant isotope of the same element?
A) boiling point is 2403 °C
B) atomic number is 31
C) isotopic mass is 69.92 amu
D) reacts with chlorine to give the compound XCl3
A) boiling point is 2403 °C
B) atomic number is 31
C) isotopic mass is 69.92 amu
D) reacts with chlorine to give the compound XCl3

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
40
Chlorine, which exists in nature in two isotopic forms, has an atomic mass of 35.5 amu. This means that ________.
A) all chlorine atoms have masses of 35.5 amu
B) 35.5 amu is the upper limit for the mass of a chlorine atom
C) 35Cl and 37Cl each constitute approximately 50% of all chlorine atoms
D) 35Cl constitutes 75% of full chlorine atoms, 37Cl represents 25%
A) all chlorine atoms have masses of 35.5 amu
B) 35.5 amu is the upper limit for the mass of a chlorine atom
C) 35Cl and 37Cl each constitute approximately 50% of all chlorine atoms
D) 35Cl constitutes 75% of full chlorine atoms, 37Cl represents 25%

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
41
An isotope of which element is used as the standard for the relative mass scale for atoms?
A) carbon
B) oxygen
C) hydrogen
D) helium
A) carbon
B) oxygen
C) hydrogen
D) helium

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
42
The isotopic mass for a particular isotope of gold is 196.9665 amu. The mass number for this gold isotope ________.
A) is 79
B) is 196
C) is 197
D) cannot be determined from the information given
A) is 79
B) is 196
C) is 197
D) cannot be determined from the information given

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
43
Three naturally occurring isotopes exist for the hypothetical element athenium. 2.50% of athenium atoms have a relative mass of 26.0 amu, 75.0% of athenium atoms have a relative mass of 27.0 amu, and 22.5% of athenium atoms have a relative mass of 28.0 amu. What is the atomic mass of athenium?
A) 26.8 amu
B) 27.0 amu
C) 28.0 amu
D) 27.2 amu
A) 26.8 amu
B) 27.0 amu
C) 28.0 amu
D) 27.2 amu

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
44
Element A exists in three isotopic forms with masses of 21.0, 25.0 and 26.0 amu respectively. Element B also exists in three isotopic forms with masses of 22.0, 24.0 and 26.0 amu respectively. It is true that ________.
A) element A has a higher atomic mass than B
B) element B has a higher atomic mass than A
C) A and B have identical atomic masses since the sums of their isotopic masses are equal
D) you need the percentages of each isotope to determine their atomic masses
A) element A has a higher atomic mass than B
B) element B has a higher atomic mass than A
C) A and B have identical atomic masses since the sums of their isotopic masses are equal
D) you need the percentages of each isotope to determine their atomic masses

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
45
What is the atomic mass of B if 19.9% of all B atoms have a mass of 10.01 amu and 80.1% have a mass of 11.01 amu?
A) 10.21 amu
B) 10.50 amu
C) 10.63 amu
D) 10.81 amu
A) 10.21 amu
B) 10.50 amu
C) 10.63 amu
D) 10.81 amu

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
46
A hypothetical element, Rz, has two isotopes: 169Rz = 168.94 amu and 171Rz = 170.98 amu. Eleven of every fifteen atoms of Rz found in nature exist as the 169Rz isotope. What is the average atomic mass of the element Rz?
A) 169.48 amu
B) 168.95 amu
C) 170.56 amu
D) 171.01 amu
A) 169.48 amu
B) 168.95 amu
C) 170.56 amu
D) 171.01 amu

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
47
Three naturally occurring isotopes of carbon exist: The atomic mass of carbon is 12.01 amu. Which of the three carbon isotopes is most abundant in nature?
A) carbon-12
B) carbon-13
C) carbon-14
D) All three occur with equal abundance.
A) carbon-12
B) carbon-13
C) carbon-14
D) All three occur with equal abundance.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following was not a conclusion obtained from the gold foil-alpha particle scattering experiment?
A) An atom is mostly empty space.
B) An atom's nucleus is positively charged.
C) An atom's nucleus contains protons and neutrons.
D) Most of the mass of an atom is concentrated in its nucleus.
A) An atom is mostly empty space.
B) An atom's nucleus is positively charged.
C) An atom's nucleus contains protons and neutrons.
D) Most of the mass of an atom is concentrated in its nucleus.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
49
Which is a conclusion about the atom's structure from Rutherford's gold foil-alpha particle experiments?
A) The electrons occupy most of the total volume of an atom
B) The mass of an atom is distributed uniformly throughout the atom.
C) All alpha particles are affected the same way by the atoms in the gold foil.
D) The positive charge of the atom is uniformly distributed throughout the atom.
A) The electrons occupy most of the total volume of an atom
B) The mass of an atom is distributed uniformly throughout the atom.
C) All alpha particles are affected the same way by the atoms in the gold foil.
D) The positive charge of the atom is uniformly distributed throughout the atom.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
50
Match the molecules with the appropriate classification.
-
A) heteroatomic - polyatomic molecule
B) homoatomic - polyatomic molecule
C) heteroatomic - diatomic molecule
D) homoatomic - diatomic molecule
-

A) heteroatomic - polyatomic molecule
B) homoatomic - polyatomic molecule
C) heteroatomic - diatomic molecule
D) homoatomic - diatomic molecule

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
51
Match the molecules with the appropriate classification.
-
A) heteroatomic - polyatomic molecule
B) homoatomic - polyatomic molecule
C) heteroatomic - diatomic molecule
D) homoatomic - diatomic molecule
-

A) heteroatomic - polyatomic molecule
B) homoatomic - polyatomic molecule
C) heteroatomic - diatomic molecule
D) homoatomic - diatomic molecule

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
52
Match the molecules with the appropriate classification.
-
A) heteroatomic - polyatomic molecule
B) homoatomic - polyatomic molecule
C) heteroatomic - diatomic molecule
D) homoatomic - diatomic molecule
-

A) heteroatomic - polyatomic molecule
B) homoatomic - polyatomic molecule
C) heteroatomic - diatomic molecule
D) homoatomic - diatomic molecule

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
53
Match the molecules with the appropriate classification.
-
A) heteroatomic - polyatomic molecule
B) homoatomic - polyatomic molecule
C) heteroatomic - diatomic molecule
D) homoatomic - diatomic molecule
-

A) heteroatomic - polyatomic molecule
B) homoatomic - polyatomic molecule
C) heteroatomic - diatomic molecule
D) homoatomic - diatomic molecule

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
54
Match the molecules with the appropriate classification.
-
A) heteroatomic - polyatomic molecule
B) homoatomic - polyatomic molecule
C) heteroatomic - diatomic molecule
D) homoatomic - diatomic molecule
-

A) heteroatomic - polyatomic molecule
B) homoatomic - polyatomic molecule
C) heteroatomic - diatomic molecule
D) homoatomic - diatomic molecule

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
55
Depicted are molecules that represent the following substances: NOCl, P4, H2S, IBr, and C2H2. Identify the substances present in each box.
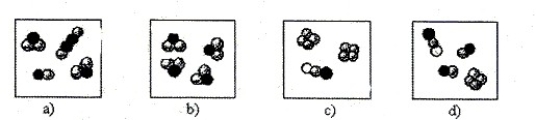


Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
56
Depicted below are molecules that represent the following substances: OF2, NH3, H2O2, CH2Cl2, and Br2. Identify the substances present in each box.
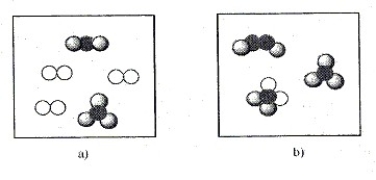


Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the terms heteroatomic, homoatomic, diatomic, triatomic, polyatomic, element and compound apply to each of the following molecules? More than one term may apply in a given situation.
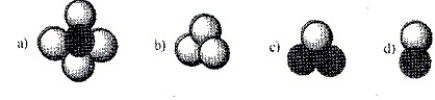


Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
58
Fill in the blanks in the following sentences using words from the following response list:
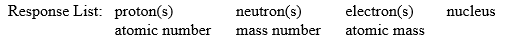
Items in the response list may be used more than once or need not be used at all.
-The __________ of an atom contains protons and __________.
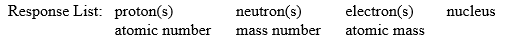
Items in the response list may be used more than once or need not be used at all.
-The __________ of an atom contains protons and __________.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
59
Fill in the blanks in the following sentences using words from the following response list:
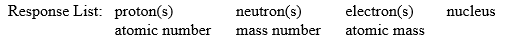
Items in the response list may be used more than once or need not be used at all.
-The identity of an atom (which element it is) is determined by the number of protons in its __________.
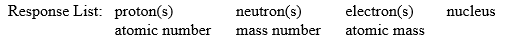
Items in the response list may be used more than once or need not be used at all.
-The identity of an atom (which element it is) is determined by the number of protons in its __________.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
60
Fill in the blanks in the following sentences using words from the following response list:
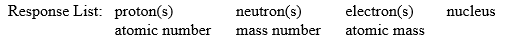
Items in the response list may be used more than once or need not be used at all.
-The atomic number gives the number of __________ and __________ in a neutral atom.
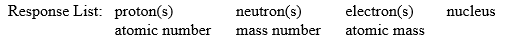
Items in the response list may be used more than once or need not be used at all.
-The atomic number gives the number of __________ and __________ in a neutral atom.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
61
Fill in the blanks in the following sentences using words from the following response list:
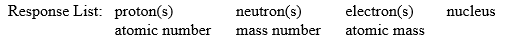
Items in the response list may be used more than once or need not be used at all.
-The mass number for an atom equals the sum of the __________ and __________ in the atom's nucleus.
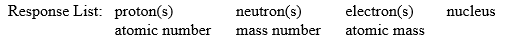
Items in the response list may be used more than once or need not be used at all.
-The mass number for an atom equals the sum of the __________ and __________ in the atom's nucleus.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
62
Fill in the blanks in the following sentences using words from the following response list:
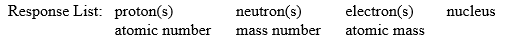
Items in the response list may be used more than once or need not be used at all.
-The mass of a __________ (a neutral species) is many times greater than the mass of an electron and is approximately the same as the mass of a __________.
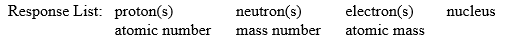
Items in the response list may be used more than once or need not be used at all.
-The mass of a __________ (a neutral species) is many times greater than the mass of an electron and is approximately the same as the mass of a __________.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
63
Fill in the blanks in the following sentences using words from the following response list:
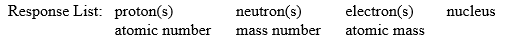
Items in the response list may be used more than once or need not be used at all.
-The __________ possesses a plus one charge, the __________ a minus one charge, and the __________ has no charge.
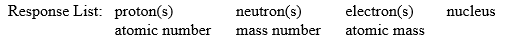
Items in the response list may be used more than once or need not be used at all.
-The __________ possesses a plus one charge, the __________ a minus one charge, and the __________ has no charge.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
64
Place in the blank the letter of the correct response in each horizontal row of choices.
-________ Number of elements in the compound Al2SO4
A) 3
B) 4
C) 5
D) 7
-________ Number of elements in the compound Al2SO4
A) 3
B) 4
C) 5
D) 7

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
65
Place in the blank the letter of the correct response in each horizontal row of choices.
-________ Number of atoms in the formula Ca(HCO3)2
A) 11
B) 15
C) 9
D) 10
-________ Number of atoms in the formula Ca(HCO3)2
A) 11
B) 15
C) 9
D) 10

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
66
Place in the blank the letter of the correct response in each horizontal row of choices.
-________ Number of neutrons in
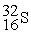
A) 15
B) 17
C) 20
D) 16
-________ Number of neutrons in
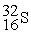
A) 15
B) 17
C) 20
D) 16

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
67
Place in the blank the letter of the correct response in each horizontal row of choices.
-________ Number of electrons in
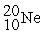
A) 20
B) 10
C) 30
D) 12
-________ Number of electrons in
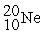
A) 20
B) 10
C) 30
D) 12

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
68
Place in the blank the letter of the correct response in each horizontal row of choices.
-________ Number of subatomic particles in
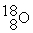
A) 26
B) 8
C) 18
D) 22
-________ Number of subatomic particles in
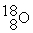
A) 26
B) 8
C) 18
D) 22

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
69
Place in the blank the letter of the correct response in each horizontal row of choices.
-________ Number of protons in
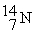
A) 7
B) 8
C) 14
D) 20
-________ Number of protons in
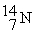
A) 7
B) 8
C) 14
D) 20

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
70
Place in the blank the letter of the correct response in each horizontal row of choices.
-________ Identity of X in
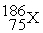
A) Ti
B) Cr
C) I
D) Re
-________ Identity of X in
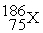
A) Ti
B) Cr
C) I
D) Re

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
71
Place in the blank the letter of the correct response in each horizontal row of choices.
-________ Mass number of a K isotope containing 21 neutrons
A) 19
B) 21
C) 40
D) 28
-________ Mass number of a K isotope containing 21 neutrons
A) 19
B) 21
C) 40
D) 28

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
72
Place in the blank the letter of the correct response in each horizontal row of choices.
-________ Mass number of an isotope containing 45 protons and 58 neutrons
A) 13
B) 45
C) 58
D) 103
-________ Mass number of an isotope containing 45 protons and 58 neutrons
A) 13
B) 45
C) 58
D) 103

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
73
Place in the blank the letter of the correct response in each horizontal row of choices.
-________ An isobar of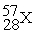
A)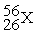
B)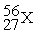
C)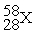
D)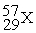
-________ An isobar of
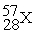
A)
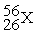
B)
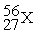
C)
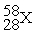
D)
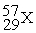

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
74
Place in the blank the letter of the correct response in each horizontal row of choices.
-________ An atom of Cu containing an equal number of protons, neutrons and electrons
A)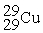
B)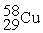
C)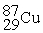
D )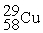
-________ An atom of Cu containing an equal number of protons, neutrons and electrons
A)
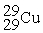
B)
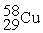
C)
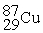
D )
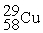

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
75
Place in the blank the letter of the correct response in each horizontal row of choices.
-________ Nuclear charge of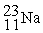
A) +10
B) +12
C) +11
D) +23
-________ Nuclear charge of
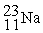
A) +10
B) +12
C) +11
D) +23

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
76
For each description on the left select the correct atom from the response list on the right. Responses may be used more than once or need not be used at all.
-________ Has a mass number of 80
A)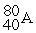
B)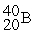
C)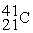
D)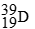
-________ Has a mass number of 80
A)
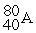
B)
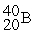
C)
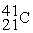
D)
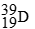

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
77
For each description on the left select the correct atom from the response list on the right. Responses may be used more than once or need not be used at all.
-________ Has an equal number of protons and neutrons
A)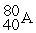
B)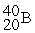
C)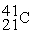
D)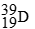
-________ Has an equal number of protons and neutrons
A)
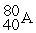
B)
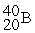
C)
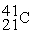
D)
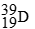

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
78
For each description on the left select the correct atom from the response list on the right. Responses may be used more than once or need not be used at all.
-________ Has more electrons than neutrons
A)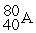
B)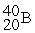
C)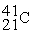
D)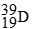
-________ Has more electrons than neutrons
A)
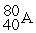
B)
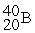
C)
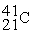
D)
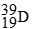

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
79
For each description on the left select the correct atom from the response list on the right. Responses may be used more than once or need not be used at all.
-________ Has more neutrons than electrons
A)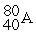
B)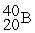
C)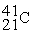
D)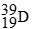
-________ Has more neutrons than electrons
A)
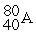
B)
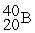
C)
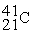
D)
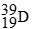

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
80
Fill in the chart below for each of the following isotopes. Be careful there may be more than one ion present.
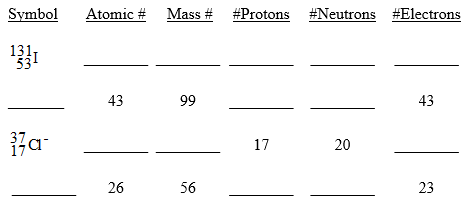


Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck


