Deck 5: Structure and Preparation of Alkenes: Elimination Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/18
Play
Full screen (f)
Deck 5: Structure and Preparation of Alkenes: Elimination Reactions
1
Carbon-carbon double bonds do not freely rotate like carbon-carbon single bonds. Why?
A) The double bond is much stronger and thus more difficult to rotate.
B) Overlap of the two 2 p orbitals of the bond would be lost.
C) The shorter bond length of the double bond makes it more difficult for the attached groups to pass each other.
D) Overlap of the orbitals of the carbon-carbon bond would be lost.
A) The double bond is much stronger and thus more difficult to rotate.
B) Overlap of the two 2 p orbitals of the bond would be lost.
C) The shorter bond length of the double bond makes it more difficult for the attached groups to pass each other.
D) Overlap of the orbitals of the carbon-carbon bond would be lost.
Overlap of the two 2 p orbitals of the bond would be lost.
2
What is the IUPAC name of the following compound?

A) 2-methyl-3-propyl-2-pentene
B) 3-ethyl-2-methyl-2-hexene
C) 4-ethyl-5-methyl-4-hexene
D) 4-methyl-3-propyl-3-pentene

A) 2-methyl-3-propyl-2-pentene
B) 3-ethyl-2-methyl-2-hexene
C) 4-ethyl-5-methyl-4-hexene
D) 4-methyl-3-propyl-3-pentene
3-ethyl-2-methyl-2-hexene
3
Which of the following alkenes exhibit E-Z isomerism?
I.
II.
III.
IV.
A) I and II
B) I and III
C) II and IV
D) I, II, and III
I.
II.
III.
IV.
A) I and II
B) I and III
C) II and IV
D) I, II, and III
I and III
4
Which of the following isomers has the highest heat of combustion?
A) 1-hexene
B) trans-3-hexene
C) cis-3-hexene
D) 2-methyl-2-pentene
A) 1-hexene
B) trans-3-hexene
C) cis-3-hexene
D) 2-methyl-2-pentene

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
5
Identify the major organic product expected from the acid-catalyzed dehydration of 2-methyl-2pentanol.
A) 2-methyl-1-pentene
B) 2-methyl-2-pentene
C) 3-methyl-1-pentene
D) cis-3-methyl-2-pentene
A) 2-methyl-1-pentene
B) 2-methyl-2-pentene
C) 3-methyl-1-pentene
D) cis-3-methyl-2-pentene

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
6
What is the slow, rate-determining step, in the acid-catalyzed dehydration of 2-methyl-2-propanol?

A) Protonation of the alcohol to form an oxonium ion.
B) Loss of water from the oxonium ion to form a carbocation.
C) Loss of a -hydrogen from the carbocation to form an alkene.
D) The simultaneous loss of a -hydrogen and water from the oxonium ion.

A) Protonation of the alcohol to form an oxonium ion.
B) Loss of water from the oxonium ion to form a carbocation.
C) Loss of a -hydrogen from the carbocation to form an alkene.
D) The simultaneous loss of a -hydrogen and water from the oxonium ion.

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following carbocations is(are) likely to undergo a rearrangement?

A) only I
B) I and III
C) II and III
D) I, II, and III

A) only I
B) I and III
C) II and III
D) I, II, and III

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
8
Predict the major product of the following reaction.
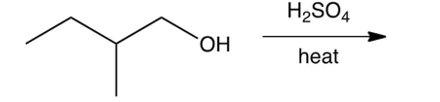
A)

B)

C)

D)


A)

B)

C)

D)


Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
9
In the dehydrohalogenation of 2-bromobutane, which conformation below leads directly to the formation of cis-2-butene? 
A) only I
B) only II
C) only III
D) I and II

A) only I
B) only II
C) only III
D) I and II

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following cannot undergo an E2 reaction?
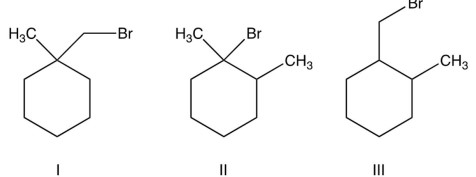
A) only I
B) only II
C) only III
D) I and III

A) only I
B) only II
C) only III
D) I and III

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following would have the fastest rate of reaction to form 4-tert-butylcyclohexene?
A)

B)

C)

D)

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
12
What is the first step in the mechanism of the dehydration reaction of a tertiary alcohol with sulfuric acid to form an alkene?
A) the loss of to form a carbocation
B) the protonation of the hydroxyl group
C) the loss of the proton from the hydroxyl group to give an alkoxide ion
D) the removal of a -hydrogen from the alcohol
A) the loss of to form a carbocation
B) the protonation of the hydroxyl group
C) the loss of the proton from the hydroxyl group to give an alkoxide ion
D) the removal of a -hydrogen from the alcohol

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following does not give 1, 2-dimethylcyclohexene as one of the acid-catalyzed dehydration products?
A)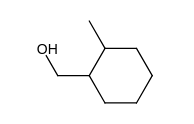
B)
C)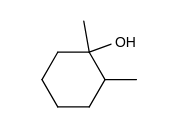
D)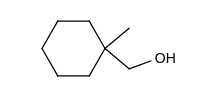
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following compounds gives a single E2 product on reaction with sodium ethoxide, ?

A) I and II
B) I and III
C) II and III
D) I, II, and III

A) I and II
B) I and III
C) II and III
D) I, II, and III

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following stereoisomers gives the exclusive E2 product shown?

A)

B)

C)

D)
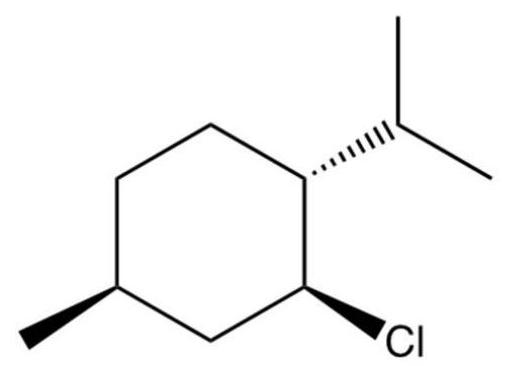

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
16
What is the major product of the reaction sequence shown below?
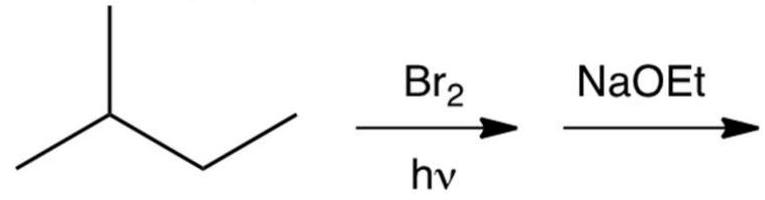
A) 2-methyl-1-butene
B) 2-methyl-2-butene
C) 3-methyl-1-butene
D) 2-methylbutane

A) 2-methyl-1-butene
B) 2-methyl-2-butene
C) 3-methyl-1-butene
D) 2-methylbutane

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the alkenes below has the Z-configuration?
A)

B)

C)

D)

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
18
If you wanted to make compound III, starting with compound I or II, what would you do?

A) React I with .
B) React II with NaOEt.
C) Either of the reactions in A or B above would work.
D) Neither of the reactions in A or B above would work.

A) React I with .
B) React II with NaOEt.
C) Either of the reactions in A or B above would work.
D) Neither of the reactions in A or B above would work.

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck


