Deck 6: The Reactions of Alkenes the Stereochemistry of Addition Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/10
Play
Full screen (f)
Deck 6: The Reactions of Alkenes the Stereochemistry of Addition Reactions
1
Which of the following compounds reacts most rapidly with HCl?
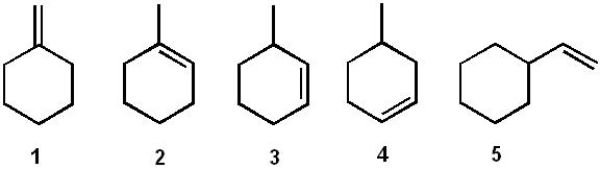
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5
1
2
Predict the major product of the reaction of 1-methylcyclohexene + HBr.
A) 1-bromo-2-methylcyclohexene
B) 1-bromo-1-methylcyclohexane
C) 2-bromo-1-methylcyclohexane
D) bromocyclohexane
E) 1-methylcyclohexene
A) 1-bromo-2-methylcyclohexene
B) 1-bromo-1-methylcyclohexane
C) 2-bromo-1-methylcyclohexane
D) bromocyclohexane
E) 1-methylcyclohexene
1-bromo-1-methylcyclohexane
3
Which set of reagents would be the best choice for the transformation shown below? 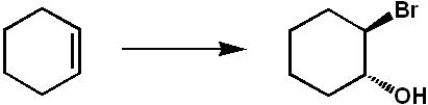
A) Br2/CH3OH
B) Br2/H2O
C) 1. Br2 2. H2O
D) 1. Br2 2. NaOH
E) 1. BH3 2. Br2 3. NaOH, H2O2, H2O

A) Br2/CH3OH
B) Br2/H2O
C) 1. Br2 2. H2O
D) 1. Br2 2. NaOH
E) 1. BH3 2. Br2 3. NaOH, H2O2, H2O
Br2/H2O
4
Assuming that a single carbocation rearrangement occurs, which of the following molecules is the major product of this reaction?
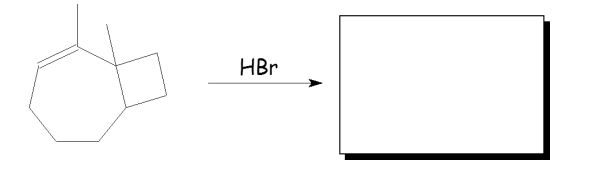
A)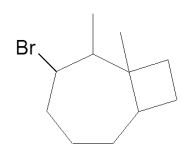
B)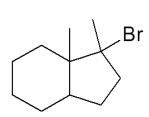
C)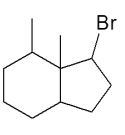
D)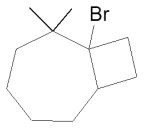
E)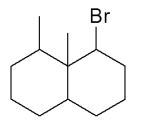

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
5
Which set of reagents would be the best choice to accomplish the transformation shown below? 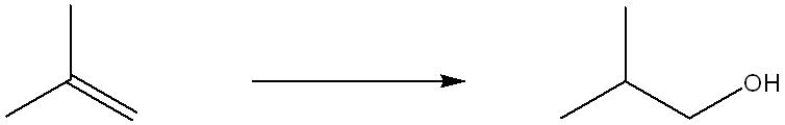
A) H2O, H2SO4
B) CH3OH, H2SO4
C) H2O/H2O2/NaOH
D) 1. BH3/THF 2. HO-,H2O2, H2O
E) 1. NaBH4 2. H3O+

A) H2O, H2SO4
B) CH3OH, H2SO4
C) H2O/H2O2/NaOH
D) 1. BH3/THF 2. HO-,H2O2, H2O
E) 1. NaBH4 2. H3O+

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
6
How many and what type of isomers are formed in the following reaction: 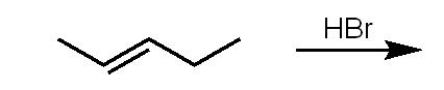
A) 2 - 2 constitutional isomers
B) 2 - 1 pair of enantiomers
C) 3 - 1 pair of enantiomers and
1 constitutional isomer
D) 3 - 1 pair of enantiomers and 1 meso compound
E) 4 - 2 pairs of enantiomers

A) 2 - 2 constitutional isomers
B) 2 - 1 pair of enantiomers
C) 3 - 1 pair of enantiomers and
1 constitutional isomer
D) 3 - 1 pair of enantiomers and 1 meso compound
E) 4 - 2 pairs of enantiomers

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
7
What is the systematic name for the epoxide shown here?
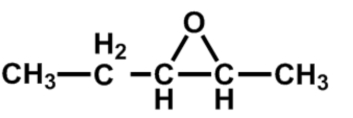
A) 1-methyl-2-ethyloxide
B) 1-ethyl-2-methyloxirane
C) 2-ethyl-3-methyloxirane
D) 2,3-epoxypentane
E) C and D

A) 1-methyl-2-ethyloxide
B) 1-ethyl-2-methyloxirane
C) 2-ethyl-3-methyloxirane
D) 2,3-epoxypentane
E) C and D

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following molecules is a possible starting material in this ozonolysis reaction? 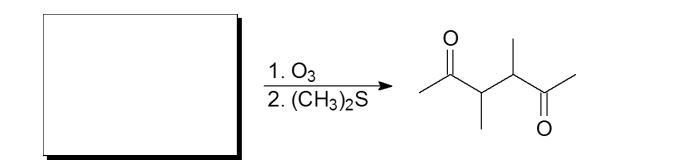
A)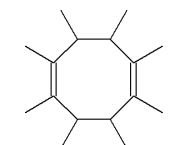
B)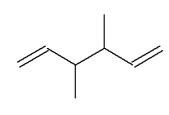
C)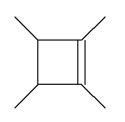
D)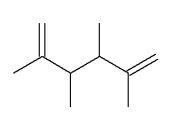
E) molecules A, C, and D

A)

B)

C)

D)

E) molecules A, C, and D

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements is true about a stereoselective reaction?
A) It forms more of one constitutional isomer than of another.
B) It forms more of one stereoisomer isomer than another.
C) Each stereoisomer forms a different stereoisomeric product or a different set of stereoisomeric products.
D) A stereoselective reaction must be stereospecific.
E) all of the above
A) It forms more of one constitutional isomer than of another.
B) It forms more of one stereoisomer isomer than another.
C) Each stereoisomer forms a different stereoisomeric product or a different set of stereoisomeric products.
D) A stereoselective reaction must be stereospecific.
E) all of the above

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
10
In the following reaction, what is the configuration of the products? 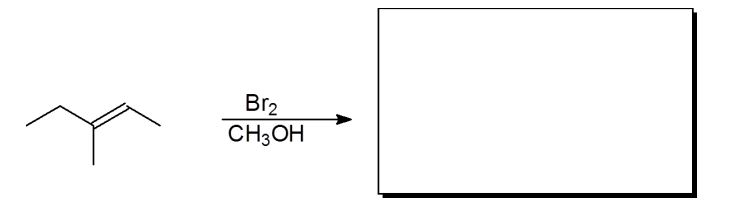
A) 2R 3R and 2R 3S
B) 2S 3S and 2R 3S
C) 2R 3R and 2S 3S
D) 2S 3R and 2R 3S
E) No stereoisomers form.

A) 2R 3R and 2R 3S
B) 2S 3S and 2R 3S
C) 2R 3R and 2S 3S
D) 2S 3R and 2R 3S
E) No stereoisomers form.

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck


