Deck 3: An Introduction to Organic Compounds Nomenclature, Physical Properties, and Structure
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/10
Play
Full screen (f)
Deck 3: An Introduction to Organic Compounds Nomenclature, Physical Properties, and Structure
1
How many of the nine constitutional isomers of heptane lack a secondary carbon?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4
1
2
Which of the following names is correct? 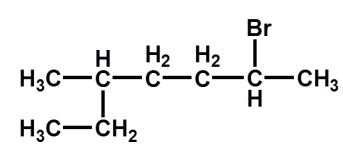
A) 2-bromo-5-ethylhexane
B) 2-ethyl-5-bromohexane
C) 5-ethyl-2-bromohexane
D) 2-bromo-5-methylheptane
E) 5-methyl-2-bromoheptane

A) 2-bromo-5-ethylhexane
B) 2-ethyl-5-bromohexane
C) 5-ethyl-2-bromohexane
D) 2-bromo-5-methylheptane
E) 5-methyl-2-bromoheptane
2-bromo-5-methylheptane
3
Choose the best name for the following compound.
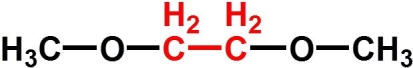
A) dimethoxyethyl ether
B) dimethoxyethane
C) 1,2-dimethoxyethane
D) 1,3-dioxobutane
E) 2-methoxyethyl methyl ether
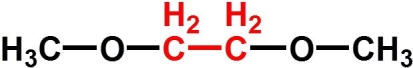
A) dimethoxyethyl ether
B) dimethoxyethane
C) 1,2-dimethoxyethane
D) 1,3-dioxobutane
E) 2-methoxyethyl methyl ether
1,2-dimethoxyethane
4
Choose the best name for the following compound. 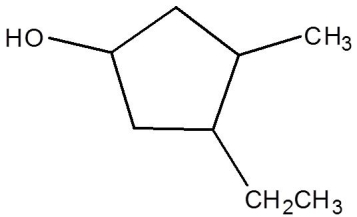
A) 1-ethyl-2-methylcyclopentanol
B) 1-ethyl-2-methyl-3-cyclopentanol
C) 3-methyl-4-ethylcyclopentanol
D) 4-ethyl-3-methylcyclopentanol
E) 3-ethyl-4-methylcyclopentanol

A) 1-ethyl-2-methylcyclopentanol
B) 1-ethyl-2-methyl-3-cyclopentanol
C) 3-methyl-4-ethylcyclopentanol
D) 4-ethyl-3-methylcyclopentanol
E) 3-ethyl-4-methylcyclopentanol

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
5
Choose the best name for the following compound.
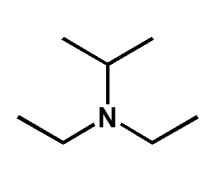
A) N,N,N-diethylisopropylamine
B) N,N-diethyl-2-propanamine
C) diethyl-2-propanamine
D) diethyl-N-propanamine
E) N,N-diethylpropylamine

A) N,N,N-diethylisopropylamine
B) N,N-diethyl-2-propanamine
C) diethyl-2-propanamine
D) diethyl-N-propanamine
E) N,N-diethylpropylamine

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is a tertiary amine?
A)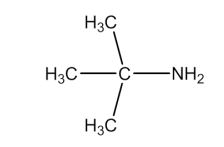
B)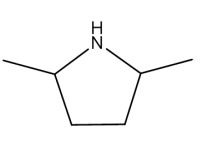
C)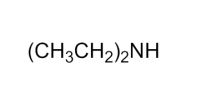
D)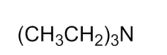
E)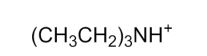
A)

B)

C)
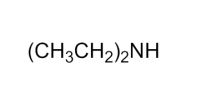
D)
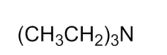
E)
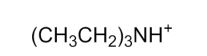

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following trimethylcyclohexanes has the most stable chair conformer?
A)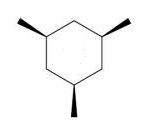
B)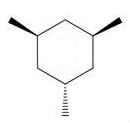
C)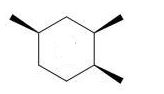
D)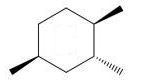
E)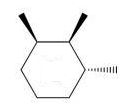
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following dimethylcyclohexanes has the most stable chair conformer?
A)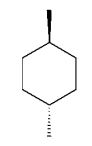
B)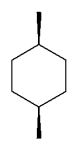
C)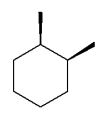
D)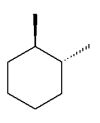
E)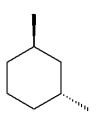
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
9
Why, primarily, does tetrahydrofuran (THF, bp 66 °C) have a higher boiling point than cyclopentane (bp 49 °C)? 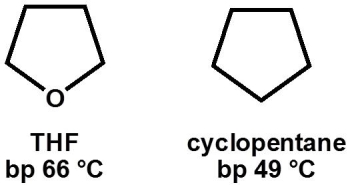
A) increased London dispersion forces
B) hydrogen bonding
C) increased mass
D) decreased London dispersion forces
E) dipole-dipole interactions

A) increased London dispersion forces
B) hydrogen bonding
C) increased mass
D) decreased London dispersion forces
E) dipole-dipole interactions

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
10
Rank the alcohols, all of similar molecular mass, in order of decreasing boiling point.
1 CH3CH2CH2CH2OH
2 CH3CH2(CH3)CHOH
3 (CH3)3COH
4 HOCH2CH2CH2OH
A) 1>2>3>4
B) 4>3>2>1
C) 3>2>1>4
D)4>1>2>3
E) 1>2>4>3
1 CH3CH2CH2CH2OH
2 CH3CH2(CH3)CHOH
3 (CH3)3COH
4 HOCH2CH2CH2OH
A) 1>2>3>4
B) 4>3>2>1
C) 3>2>1>4
D)4>1>2>3
E) 1>2>4>3

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck


