Deck 12: Radicals
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/10
Play
Full screen (f)
Deck 12: Radicals
1
What is the first step in the radical halogenation of an alkane?
A) abstraction
B) initiation
C) propagation
D) combination
E) termination
A) abstraction
B) initiation
C) propagation
D) combination
E) termination
initiation
2
Which of the following radicals is the most stable?
A)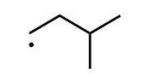
B)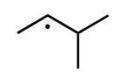
C)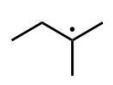
D)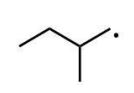
E)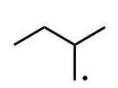
A)

B)

C)

D)

E)


3
Calculate the percentage yield of the product below formed by radical bromination. [Relative reactivities: 1°(1), 2°(82), 3°(1600)] ![<strong>Calculate the percentage yield of the product below formed by radical bromination. [Relative reactivities: 1°(1), 2°(82), 3°(1600)] </strong> A) 5% B) 15% C) 29% D) 41% E) 71%](https://storage.examlex.com/TBP1106/11ef27fc_c1bc_2773_aec8_e9f0a9aec25a_TBP1106_00.jpg)
A) 5%
B) 15%
C) 29%
D) 41%
E) 71%
![<strong>Calculate the percentage yield of the product below formed by radical bromination. [Relative reactivities: 1°(1), 2°(82), 3°(1600)] </strong> A) 5% B) 15% C) 29% D) 41% E) 71%](https://storage.examlex.com/TBP1106/11ef27fc_c1bc_2773_aec8_e9f0a9aec25a_TBP1106_00.jpg)
A) 5%
B) 15%
C) 29%
D) 41%
E) 71%
71%
4
Of the following ethers, which would be least likely to form peroxides and hence be safer to use in a laboratory?
A)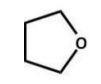
B)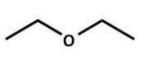
C)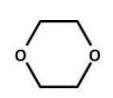
D)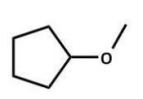
E)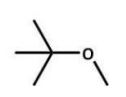
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
5
Which compound will be the major product of the following radical
Addition reaction?
A)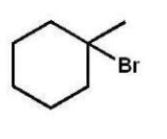
B)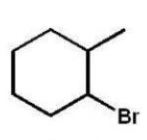
C)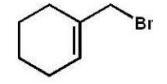
D)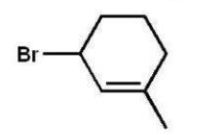
E)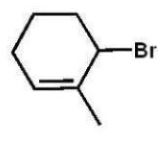
Addition reaction?

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
6
How many isomers are formed in the radical addition of HBr to 1-methylcyclohexene?
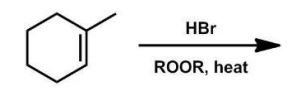
A) 1
B) 2
C) 3
D) 4
E) 6
Two new asymmetric centers are created in the product.

A) 1
B) 2
C) 3
D) 4
E) 6
Two new asymmetric centers are created in the product.

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
7
How many monobrominated constitutional isomers are possible for the following reaction? 
A) 2
B) 3
C) 4
D)5
E) 6

A) 2
B) 3
C) 4
D)5
E) 6

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
8
Rank the radicals in order from most stable to least stable. 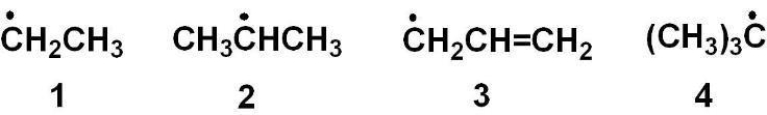
A) 1>2>3>4
B) 4>3>2>1
C) 3>4>2>1
D) 1>2>4>3
E) 4>2>3>1

A) 1>2>3>4
B) 4>3>2>1
C) 3>4>2>1
D) 1>2>4>3
E) 4>2>3>1

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
9
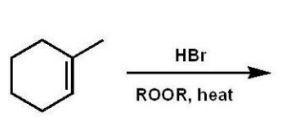
Which compound will give one product upon radical bromination using NBS, ROOR, and heat?
A)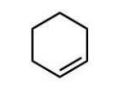
B)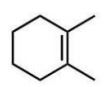
C)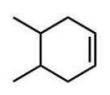
D)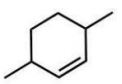
E)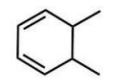
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
10
Radical inhibitors ____________.
A) prevent oxidation reactions
B) form less reactive radicals
C) destroy reactive radicals
D) include hydroquinones and vitamins A and E
E) do all of the above
A) prevent oxidation reactions
B) form less reactive radicals
C) destroy reactive radicals
D) include hydroquinones and vitamins A and E
E) do all of the above

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck


