Deck 10: Reactions of Alcohols, Ethers,epoxides, Amines, and Sulfur-Containing Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/10
Play
Full screen (f)
Deck 10: Reactions of Alcohols, Ethers,epoxides, Amines, and Sulfur-Containing Compounds
1
Which of the following statements is not true?
A) Primary, secondary, and tertiary alcohols all undergo nucleophilic substitution with HI, HBr, and HCl.
B) Carbocation stability: 1° > 2° > 3°
C) Tertiary alcohols undergo SN1 reactions with hydrogen halides.
D) Primary alcohols undergo SN2 reactions with hydrogen halides.
E) An alcohol has a strongly basic leaving group that cannot be displaced by a nucleophile.
A) Primary, secondary, and tertiary alcohols all undergo nucleophilic substitution with HI, HBr, and HCl.
B) Carbocation stability: 1° > 2° > 3°
C) Tertiary alcohols undergo SN1 reactions with hydrogen halides.
D) Primary alcohols undergo SN2 reactions with hydrogen halides.
E) An alcohol has a strongly basic leaving group that cannot be displaced by a nucleophile.
Carbocation stability: 1° > 2° > 3°
2
Which alkene is the major product of this dehydration?
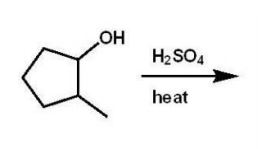
A)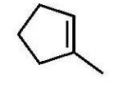
B)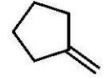
C)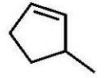
D)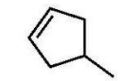
E)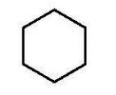

A)

B)

C)

D)

E)


3
Using reagents such as SOCl2 or PBr3, alcohols are converted into alkyl halides. This approach ____________.
A) inverts the configuration if the OH group is bonded to an asymmetric center
B) converts the OH group into a better leaving group
C) works well for 1° and 2° alcohols
D) creates a leaving group that is a weaker base than the halide ion
E) does all of the above
A) inverts the configuration if the OH group is bonded to an asymmetric center
B) converts the OH group into a better leaving group
C) works well for 1° and 2° alcohols
D) creates a leaving group that is a weaker base than the halide ion
E) does all of the above
does all of the above
4
In the reaction of the following alcohol, what type of reaction is involved in the transformation? 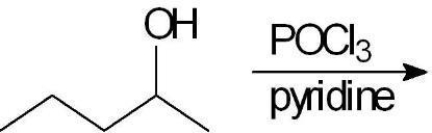
A) SN1
B) SN2
C) E1
D) E2
E) an anti-Zaitzev elimination

A) SN1
B) SN2
C) E1
D) E2
E) an anti-Zaitzev elimination

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
5
Which set of reaction conditions will produce the indicated product?

A) 1. TsCl/pyridine 2. NaCN
B) PBr3/pyridine 2. NaCN
C) SOCl2/pyridine 2. NaCN
D) PCl3/pyridine 2. NaCN
E) B, C, and D will all work.

A) 1. TsCl/pyridine 2. NaCN
B) PBr3/pyridine 2. NaCN
C) SOCl2/pyridine 2. NaCN
D) PCl3/pyridine 2. NaCN
E) B, C, and D will all work.

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following reagents can be used to prepare an aldehyde from a 1° alcohol?
A) 1. DMSO/oxalyl chloride (COCl)2 2. (Et)3N
B) Na2Cr2O7/H2SO4/H2O
C) PCC/pyridine/CH2Cl2
D) NaOCl/acetic acid
E) A, C, and D
A) 1. DMSO/oxalyl chloride (COCl)2 2. (Et)3N
B) Na2Cr2O7/H2SO4/H2O
C) PCC/pyridine/CH2Cl2
D) NaOCl/acetic acid
E) A, C, and D

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
7
Which alcohol will not undergo a chromic acid oxidation?
A)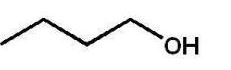
B)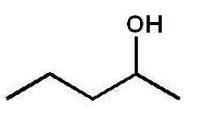
C)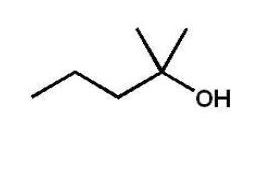
D)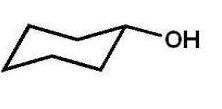
E)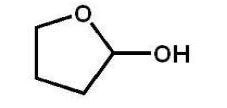
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
8
What is the major product of this reaction?
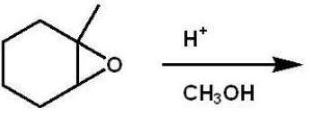
A)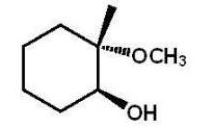
B)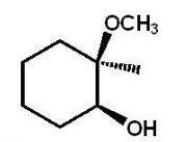
C)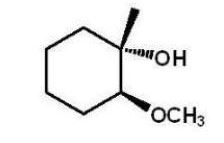
D)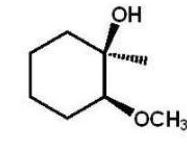
E) A and B

A)

B)

C)

D)

E) A and B

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
9
What is the major product of the following reaction? 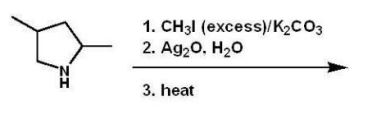
A)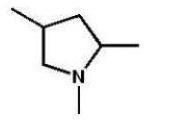
B)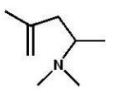
C)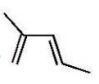
D)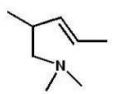
E)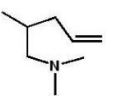

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
10
SAM is a very good methylating agent in biological systems because the transferred methyl group
____________.
A) was attached to a large molecule
B) was attached to a water-soluble molecule
C) occurred in a polar protic solvent
D) was attached to a sulfonium ion
E) None of the above are true.
____________.
A) was attached to a large molecule
B) was attached to a water-soluble molecule
C) occurred in a polar protic solvent
D) was attached to a sulfonium ion
E) None of the above are true.

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck


