Deck 2: Science Fiction, Bad Science, and Pseudoscience:
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/53
Play
Full screen (f)
Deck 2: Science Fiction, Bad Science, and Pseudoscience:
1
What kind of bond holds two water molecules to each other?
A) hydrogen bonds
B) ionic bonds
C) nonpolar covalent bonds
D) polar covalent bonds
A) hydrogen bonds
B) ionic bonds
C) nonpolar covalent bonds
D) polar covalent bonds
A
2
Which macromolecule has a sugar-phosphate backbone?
A) lipid
B) nucleic acid
C) protein
D) polysaccharide
A) lipid
B) nucleic acid
C) protein
D) polysaccharide
B
3
Which chemical condition describes the electrons in a water molecule being shared unequally between the hydrogen and oxygen atoms?
A) hydrophobic
B) ionic
C) noncovalent
D) polar
A) hydrophobic
B) ionic
C) noncovalent
D) polar
D
4
Which term best describes the sugar in a sugarwater solution?
A) product
B) reactant
C) solute
D) solvent
A) product
B) reactant
C) solute
D) solvent

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
5
Imagine a newly discovered biological molecule that is mostly hydrophobic in its structure. How would this new molecule be classified?
A) carbohydrate
B) lipid
C) nucleic acid
D) protein
A) carbohydrate
B) lipid
C) nucleic acid
D) protein

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
6
Which feature is present in all known living things?
A) genes made from proteins
B) growth from inorganic materials
C) absorption of sunlight for energy
D) metabolic reactions
A) genes made from proteins
B) growth from inorganic materials
C) absorption of sunlight for energy
D) metabolic reactions

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following pairs of molecules can be held together by a hydrogen bond?
A) one polar molecule and one nonpolar molecule
B) two negative ions
C) two nonpolar molecules
D) two polar molecules
A) one polar molecule and one nonpolar molecule
B) two negative ions
C) two nonpolar molecules
D) two polar molecules

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
8
Which characteristic necessary for life would exclude zombies from being a living thing?
A) consumption of energy-containing molecules
B) fully functioning homeostatic abilities
C) reproduction of more zombies
D) response to external stimuli
A) consumption of energy-containing molecules
B) fully functioning homeostatic abilities
C) reproduction of more zombies
D) response to external stimuli

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
9
What is the name of a molecule composed of one or more sugars?
A) carbohydrate
B) lipid
C) nucleic acid
D) polypeptide
A) carbohydrate
B) lipid
C) nucleic acid
D) polypeptide

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
10
Which component of amino acids accounts for the differences in their properties?
A) the amino group
B) the carboxyl group
C) the side group
D) the type of bonds
A) the amino group
B) the carboxyl group
C) the side group
D) the type of bonds

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following are found in the nucleus of an atom?
A) electrons and neutrons
B) neutrons and protons
C) protons and electrons
D) neutrons, electrons, and protons
A) electrons and neutrons
B) neutrons and protons
C) protons and electrons
D) neutrons, electrons, and protons

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
12
What molecule is composed only of chains and rings of hydrogen and carbon?
A) carbohydrate
B) hydrocarbon
C) polypeptide
D) polysaccharide
A) carbohydrate
B) hydrocarbon
C) polypeptide
D) polysaccharide

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
13
Which element is the basis for the macromolecules found in living things?
A) carbon
B) hydrogen
C) nitrogen
D) oxygen
A) carbon
B) hydrogen
C) nitrogen
D) oxygen

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
14
When would an atom be least likely to form chemical bonds with other atoms?
A) The number of protons equals the number of electrons.
B) The number of protons equals the number of neutrons.
C) There is only one electron in the valence shell.
D) The valence shell is full of electrons.
A) The number of protons equals the number of electrons.
B) The number of protons equals the number of neutrons.
C) There is only one electron in the valence shell.
D) The valence shell is full of electrons.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
15
Which term best describes the water in a sugarwater solution?
A) product
B) reactant
C) solute
D) solvent
A) product
B) reactant
C) solute
D) solvent

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
16
What kind of bond is found between the individual atoms of a single water molecule?
A) hydrogen bonds
B) ionic bonds
C) covalent bonds that are not polar
D) covalent bonds that are polar
A) hydrogen bonds
B) ionic bonds
C) covalent bonds that are not polar
D) covalent bonds that are polar

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
17
How do the concentrations of H⁺ and OH− compare to each other in an acidic solution?
A) The concentration of H⁺ is higher.
B) The concentration of H⁺ is lower.
C) The concentration of H⁺ is the same.
D) Acidic solutions do not contain H⁺.
A) The concentration of H⁺ is higher.
B) The concentration of H⁺ is lower.
C) The concentration of H⁺ is the same.
D) Acidic solutions do not contain H⁺.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
18
The number of which subatomic particle designates the atomic number of an element?
A) electrons
B) neutrons
C) protons
D) protons plus neutrons
A) electrons
B) neutrons
C) protons
D) protons plus neutrons

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
19
What type of chemical bond connects the complementary strands of a DNA molecule to each other?
A) hydrogen bonds
B) ionic bonds
C) nonpolar covalent bonds
D) polar covalent bonds
A) hydrogen bonds
B) ionic bonds
C) nonpolar covalent bonds
D) polar covalent bonds

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
20
What is the pH of a neutral solution?
A) 1
B) 5
C) 7
D) 9
A) 1
B) 5
C) 7
D) 9

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
21
Which monomer units combine to form proteins?
A) amino acids
B) fatty acids
C) nucleotides
D) sugars
A) amino acids
B) fatty acids
C) nucleotides
D) sugars

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
22
What are all of the chemical processes that occur in the cells of an organism considered?

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
23
Why is oxygen considered highly electronegative?
A) It pulls electrons toward itself.
B) It has a nonpolar structure.
C) It repels electrons away from its nucleus.
D) It discharges electrons readily out of the atom.
A) It pulls electrons toward itself.
B) It has a nonpolar structure.
C) It repels electrons away from its nucleus.
D) It discharges electrons readily out of the atom.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
24
What are the monomer units that compose proteins? (two words)

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
25
Which component of cholesterol would classify it as a lipid?
A) carbohydrates
B) phosphates
C) glycerol
D) hydrocarbons
A) carbohydrates
B) phosphates
C) glycerol
D) hydrocarbons

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is nonpolar?
A) a positive ion
B) a negative ion
C) a neutral ion
D) a molecule with no partial charges
A) a positive ion
B) a negative ion
C) a neutral ion
D) a molecule with no partial charges

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
27
What is the name for the ability of living things to maintain a relatively constant internal environment?
A) cellular respiration
B) homeostasis
C) metabolism
D) stimulus-response
A) cellular respiration
B) homeostasis
C) metabolism
D) stimulus-response

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
28
Which monomer units combine to form polysaccharides?
A) amino acids
B) fatty acids
C) nucleotides
D) sugars
A) amino acids
B) fatty acids
C) nucleotides
D) sugars

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is an element?
A) water
B) methane
C) hydrogen
D) carbon dioxide
A) water
B) methane
C) hydrogen
D) carbon dioxide

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
30
What is the ability of living things to maintain a relatively constant internal environment even when the external environment is changing?

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is considered part of a living organism's metabolism?
A) secretion of wastes
B) growth and development
C) responses to external stimuli
D) reproduction
A) secretion of wastes
B) growth and development
C) responses to external stimuli
D) reproduction

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following has the lowest OH− concentration?
A) baking soda
B) pure water
C) coffee
D) battery acid
A) baking soda
B) pure water
C) coffee
D) battery acid

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is a lipid?
A) cellulose
B) cholesterol
C) sucrose
D) ribonucleic acid
A) cellulose
B) cholesterol
C) sucrose
D) ribonucleic acid

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
34
What type of electric charge do protons carry?
A) negative
B) positive
C) neutral
D) no charge
A) negative
B) positive
C) neutral
D) no charge

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
35
What does a neutral pH of 7 actually represent?
A) a greater number of H⁺ ions than OH− ions
B) a greater number of OH− ions than H⁺ ions
C) an equal number of H⁺ and OH− ions
D) no H⁺ and OH− ions are present
A) a greater number of H⁺ ions than OH− ions
B) a greater number of OH− ions than H⁺ ions
C) an equal number of H⁺ and OH− ions
D) no H⁺ and OH− ions are present

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
36
Which monomer units combine to form nucleic acids?
A) amino acids
B) fatty acids
C) nucleotides
D) sugars
A) amino acids
B) fatty acids
C) nucleotides
D) sugars

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
37
What are the smallest units into which an element can be broken down?

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
38
Which type of macromolecule is present in enzymes?
A) carbohydrates
B) lipids
C) nucleic acids
D) proteins
A) carbohydrates
B) lipids
C) nucleic acids
D) proteins

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
39
Sodium chloride is composed of molecules that are stable when dry. In the presence of water, however, the atoms of the molecules separate from each other. What type of chemical bond holds the dry substance together?
A) hydrogen bonds
B) ionic bonds
C) nonpolar covalent bonds
D) polar covalent bonds
A) hydrogen bonds
B) ionic bonds
C) nonpolar covalent bonds
D) polar covalent bonds

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
40
If a solution has a pH of 2, how does its H⁺ ion concentration compare to a solution with a pH of 4?
A) It is 2 times higher.
B) It is 10 times higher.
C) It is 100 times higher.
D) It is 1000 times higher.
A) It is 2 times higher.
B) It is 10 times higher.
C) It is 100 times higher.
D) It is 1000 times higher.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
41
Which feature is found in prokaryotic cells?
A) nucleus
B) endoplasmic reticulum
C) Golgi apparatus
D) cell wall
A) nucleus
B) endoplasmic reticulum
C) Golgi apparatus
D) cell wall

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
42
Which feature is found in both prokaryotic and eukaryotic cells?
A) mitochondrion
B) Golgi body
C) DNA
D) centriole
A) mitochondrion
B) Golgi body
C) DNA
D) centriole

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
43
Why might bacteria be considered more successful than humans?
A) Humans are resistant to bacterial infections.
B) Bacteria have very specialized food sources.
C) Bacteria have been evolving for billions of years.
D) Bacteria are less diversified than humans.
A) Humans are resistant to bacterial infections.
B) Bacteria have very specialized food sources.
C) Bacteria have been evolving for billions of years.
D) Bacteria are less diversified than humans.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
44
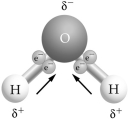
Use the figure to answer the following question. If two or more of these molecules are in proximity to one another, how will they bond together?
A) hydrogen bonding, with two hydrogen atoms bonded together
B) covalent bonding, with two oxygen atoms bonded together
C) hydrogen bonding, with a hydrogen atom bonded to an oxygen atom
D) ionic bonding, with a hydrogen ion bonded to an oxygen atom

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
45
How does the diameter of a prokaryotic cell compare to the diameter of a eukaryotic cell?
A) Prokaryotes have twice the diameter of a eukaryote.
B) Prokaryotes have one-half the diameter of a eukaryote.
C) Prokaryotes have one-tenth the diameter of a eukaryote.
D) Prokaryotes have one-thousandth the diameter of a eukaryote.
A) Prokaryotes have twice the diameter of a eukaryote.
B) Prokaryotes have one-half the diameter of a eukaryote.
C) Prokaryotes have one-tenth the diameter of a eukaryote.
D) Prokaryotes have one-thousandth the diameter of a eukaryote.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
46
What kind of molecule forms a bilayer that is the basis for all cellular membranes?
A) carbohydrate
B) cholesterol
C) phospholipid
D) protein
A) carbohydrate
B) cholesterol
C) phospholipid
D) protein

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following could accept one (and only one) electron?
A) carbon (atomic number = 6)
B) nitrogen (atomic number = 7)
C) oxygen (atomic number = 8)
D) hydrogen (atomic number = 1)
A) carbon (atomic number = 6)
B) nitrogen (atomic number = 7)
C) oxygen (atomic number = 8)
D) hydrogen (atomic number = 1)

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
48
What are the internal membrane-bound compartments found in eukaryotic cells called?

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
49
What does "hydrophobic" mean?
A) made of water
B) repelled by water
C) attracted to water
D) dissolved in water
A) made of water
B) repelled by water
C) attracted to water
D) dissolved in water

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
50
Why is the cohesion of water important for biological systems?
A) It helps to moderate the temperature of cold-blooded animals.
B) It helps transport water from the roots to the leaves of a plant.
C) It prevents chemical reactions from occurring in body systems.
D) It prevents salts from dissolving inside the plasma membrane.
A) It helps to moderate the temperature of cold-blooded animals.
B) It helps transport water from the roots to the leaves of a plant.
C) It prevents chemical reactions from occurring in body systems.
D) It prevents salts from dissolving inside the plasma membrane.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
51
According to the theory of evolution, what characteristics do all living organisms share suggesting that all life-forms on Earth arose from a single ancestor?
A) They share a common organic chemistry.
B) They have DNA within their nucleus.
C) They exhibit the same basic cell wall.
D) They perform the same mode of reproduction.
A) They share a common organic chemistry.
B) They have DNA within their nucleus.
C) They exhibit the same basic cell wall.
D) They perform the same mode of reproduction.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
52
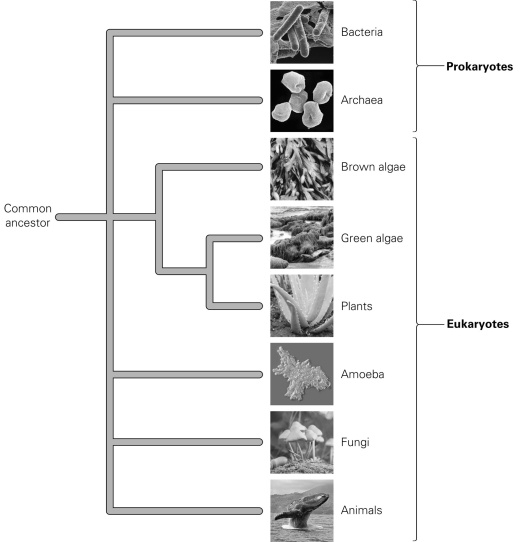
According to the diagram, which organisms are most closely related on the tree of life?
A) amoeba and brown algae
B) bacteria and animals
C) fungi and archaea
D) green algae and plants

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
53
What is the fundamental structural unit of life on Earth?

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck


