Deck 3: Amino Acids and the Primary Structures of Proteins
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/107
Play
Full screen (f)
Deck 3: Amino Acids and the Primary Structures of Proteins
1
Amino acids with non-ionizable side chains are zwitterions when they are ________.
A)in any solution
B)at physiological pH,pH = 7.4
C)in acidic solutions only
D)in alkaline solutions only
E)All of the above
A)in any solution
B)at physiological pH,pH = 7.4
C)in acidic solutions only
D)in alkaline solutions only
E)All of the above
at physiological pH,pH = 7.4
2
The amino acids in polypeptide chains which contain sulfur (S)are
A)cysteine,cystine,and methionine.
B)cystine.
C)methionine only.
D)cysteine only.
E)cysteine and methionine.
A)cysteine,cystine,and methionine.
B)cystine.
C)methionine only.
D)cysteine only.
E)cysteine and methionine.
cysteine and methionine.
3
The RS system of nomenclature describes
A)the relative sizes of molecules.
B)the way the amino acid side chains are arranged.
C)the absolute configuration about chiral carbon centers .
D)the strength of the chemical groups in amino acids.
A)the relative sizes of molecules.
B)the way the amino acid side chains are arranged.
C)the absolute configuration about chiral carbon centers .
D)the strength of the chemical groups in amino acids.
the absolute configuration about chiral carbon centers .
4
At neutral pH,the net charge of serine is
A)positive.
B)negative.
C)zero.
D)None of the above.
A)positive.
B)negative.
C)zero.
D)None of the above.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
5
The overall shape of a protein is greatly influenced by
A)amino acid R group properties.
B)charged amino acids.
C)hydrophobic amino acids.
D)pH.
E)hydrophilic amino acids.
A)amino acid R group properties.
B)charged amino acids.
C)hydrophobic amino acids.
D)pH.
E)hydrophilic amino acids.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
6
An amino acid with two chiral carbon atoms
A)is unstable.
B)can exist in 4 forms,all of which are superimposable.
C)can form three possible stereoisomers.
D)can form four possible stereoisomers.
E)can form five possible stereoisomers.
A)is unstable.
B)can exist in 4 forms,all of which are superimposable.
C)can form three possible stereoisomers.
D)can form four possible stereoisomers.
E)can form five possible stereoisomers.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
7
If the R group of an amino acid is -CH₃,then the name of this compound is
A)methyl amino acid.
B)2-aminopropanoic acid.
C)alanine.
D)All of the above.
E)B and C.
A)methyl amino acid.
B)2-aminopropanoic acid.
C)alanine.
D)All of the above.
E)B and C.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
8
Amino acids found in meteorites and near stars are
A)D isomers only.
B)L isomers only.
C)Both D and L isomers.
D)not isomers.
A)D isomers only.
B)L isomers only.
C)Both D and L isomers.
D)not isomers.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
9
Amino acids are named that because each one
A)is a unique carboxylic acid.
B)has a standard configuration.
C)is a carboxyl derivative of an amide acid.
D)is an amino derivative of a carboxylic acid.
A)is a unique carboxylic acid.
B)has a standard configuration.
C)is a carboxyl derivative of an amide acid.
D)is an amino derivative of a carboxylic acid.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
10
Tyrosine and tryptophan are less hydrophobic than phenylalanine because
A)phenylalanine has an indole group.
B)phenylalanine has no polar group in the side chain.
C)phenylalanine is a phenol.
D)tyrosine and tryptophan have smaller R groups.
E)All of the above.
A)phenylalanine has an indole group.
B)phenylalanine has no polar group in the side chain.
C)phenylalanine is a phenol.
D)tyrosine and tryptophan have smaller R groups.
E)All of the above.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
11
The R group of an amino acid determines if it is
A)hydrophilic or hydrophobic.
B)polar or nonpolar.
C)charged or uncharged.
D)an acid or a base.
E)All of the above.
A)hydrophilic or hydrophobic.
B)polar or nonpolar.
C)charged or uncharged.
D)an acid or a base.
E)All of the above.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
12
Glycine is not a stereoisomer because
A)it has no chiral carbon.
B)it does not form enantiomers.
C)it does not exist in two non-superimposable mirror-image forms.
D)All of the above.
E)A and B only.
A)it has no chiral carbon.
B)it does not form enantiomers.
C)it does not exist in two non-superimposable mirror-image forms.
D)All of the above.
E)A and B only.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
13
Ultraviolet (UV)light can be used to estimate protein solution concentrations because
A)phenylalanine absorbs at 260 nm.
B)all the amino acids absorb UV light.
C)aromatic amino acids absorb at 280 nm.
D)tryptophan and tyrosine absorb at 280 nm.
E)All of the above.
A)phenylalanine absorbs at 260 nm.
B)all the amino acids absorb UV light.
C)aromatic amino acids absorb at 280 nm.
D)tryptophan and tyrosine absorb at 280 nm.
E)All of the above.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
14
Concentrations of some proteins cannot be estimated by UV spectrophotometry because they are
A)low in glycine and valine.
B)low in tryptophan and tyrosine.
C)low in protein.
D)high in aromatic amino acids.
A)low in glycine and valine.
B)low in tryptophan and tyrosine.
C)low in protein.
D)high in aromatic amino acids.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
15
Alanine,valine,leucine and isoleucine are important in three dimensional structure because they
A)are branched.
B)are highly hydrophobic.
C)are highly hydrophilic.
D)attract water molecules.
A)are branched.
B)are highly hydrophobic.
C)are highly hydrophilic.
D)attract water molecules.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
16
Fossil dating by amino acid racemization measured
A)the amount of a D-amino acid present.
B)the amount of an L-amino acid present.
C)the amounts of both D and L forms of an amino acid present.
D)the total of all amino acids present.
A)the amount of a D-amino acid present.
B)the amount of an L-amino acid present.
C)the amounts of both D and L forms of an amino acid present.
D)the total of all amino acids present.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
17
Disulfide bridges can form in proteins ________.
A)only between cysteine residues side-by-side in the protein sequence
B)between cysteine residues that are close in three-dimensional space,but not necessarily close in the primary structure
C)between two cystine residues in proteins
D)between any two methionines or cysteines
A)only between cysteine residues side-by-side in the protein sequence
B)between cysteine residues that are close in three-dimensional space,but not necessarily close in the primary structure
C)between two cystine residues in proteins
D)between any two methionines or cysteines

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
18
The number of commonly found amino acids with only one chiral carbon is
A)17.
B)18.
C)19.
D)20.
A)17.
B)18.
C)19.
D)20.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
19
Proline is distinct among the 20 commonly found amino acids because
A)it is a ring compound.
B)it is hydrophilic and ionic.
C)the nitrogen of the amino group is in a ring.
D)the carbon of the carboxyl group is in a ring.
E)it has little effect on protein structure.
A)it is a ring compound.
B)it is hydrophilic and ionic.
C)the nitrogen of the amino group is in a ring.
D)the carbon of the carboxyl group is in a ring.
E)it has little effect on protein structure.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
20
The last common ancestor of modern organisms must have used
A)D amino acids.
B)L amino acids.
C)both D and L amino acids.
D)either D or L amino acids.
A)D amino acids.
B)L amino acids.
C)both D and L amino acids.
D)either D or L amino acids.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
21
Proteins can be modified by adding ________ to protein residues.
A)sugars and phosphate groups
B)hydroxyl and formyl groups
C)cystine
D)A and B
E)All of the above
A)sugars and phosphate groups
B)hydroxyl and formyl groups
C)cystine
D)A and B
E)All of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
22
Selenocysteine,N-formylmethionine and pyrrolysine are found in many proteins.These amino acids are considered standard amino acids along with 20 common amino acids because they
A)are incorporated into proteins from specific codons.
B)are formed by post-translational modifications.
C)play key roles in metabolism.
D)are precursors of other important amino acids.
A)are incorporated into proteins from specific codons.
B)are formed by post-translational modifications.
C)play key roles in metabolism.
D)are precursors of other important amino acids.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
23
Amino acids which are not incorporated into polypeptides are sometimes converted into
A)neurotransmitters.
B)methyl donors.
C)antibiotics.
D)blood flow controllers.
E)All of the above.
A)neurotransmitters.
B)methyl donors.
C)antibiotics.
D)blood flow controllers.
E)All of the above.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
24
The pH inside cells is normally near pH 7.At pH 7 which statement is true about the charges (ionization state)of the α-Carboxyl and α-Amino groups of an amino acid?
A)The α-Carboxyl group is 1- and the α-Amino group is 1+.
B)The α-Carboxyl group is 1+ and the α-Amino group is 1-.
C)The α-Carboxyl group is 1- and the α-Amino group is uncharged.
D)Both groups are uncharged (not ionizeD)at pH 7.
A)The α-Carboxyl group is 1- and the α-Amino group is 1+.
B)The α-Carboxyl group is 1+ and the α-Amino group is 1-.
C)The α-Carboxyl group is 1- and the α-Amino group is uncharged.
D)Both groups are uncharged (not ionizeD)at pH 7.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
25
Which structure below is appropriate for glycine at neutral pH?
A)H₂NCH₂COOH
B)H₂NCH₂COO-
C)+H₃NCH₂COO-
D)+H₃NCH₂COOH
A)H₂NCH₂COOH
B)H₂NCH₂COO-
C)+H₃NCH₂COO-
D)+H₃NCH₂COOH

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
26
Arginine is the most basic of the 20 amino acids because its side chain is ________ under most cell conditions.
A)very highly charged
B)hydrophobic
C)titrated
D)protonated
E)negatively charged
A)very highly charged
B)hydrophobic
C)titrated
D)protonated
E)negatively charged

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
27
A polypeptide chain may have abrupt changes in direction and restriction in geometry because of the presence of
A)arginine.
B)glycine.
C)proline.
D)leucine or isoleucine.
A)arginine.
B)glycine.
C)proline.
D)leucine or isoleucine.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
28
When cystine is isolated after a protein is hydrolyzed,it is deduced that
A)2 cysteine amino acids are present in the protein.
B)the protein is linked by a disulfide bridge.
C)2 cysteine amino acids in the protein or proteins must be adjacent.
D)All of the above.
A)2 cysteine amino acids are present in the protein.
B)the protein is linked by a disulfide bridge.
C)2 cysteine amino acids in the protein or proteins must be adjacent.
D)All of the above.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
29
At the isoelectric pH of an amino acid which has two pKa values the net charge is
A)0.5.
B)1.
C)0.
D)-1.
A)0.5.
B)1.
C)0.
D)-1.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
30
Which amino acid is ideal for the transfer of protons within the catalytic site of enzymes due to the presence of significant amounts of both the protonated and deprotonated forms of its side chain at biological pH?
A)Lysine.
B)Asparagine.
C)Tyrosine.
D)Cysteine.
E)Histidine.
A)Lysine.
B)Asparagine.
C)Tyrosine.
D)Cysteine.
E)Histidine.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
31
The pKa's of isoleucine's α-Carboxyl group and α-Amino group are 2.3 and 9.8,respectively.Calculate the isoelectric point.
A)2.3
B)6.0
C)9.8
D)The isoelectric point cannot be calculated without the pKa value for the side chain.
A)2.3
B)6.0
C)9.8
D)The isoelectric point cannot be calculated without the pKa value for the side chain.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
32
Although the hydroxyl groups in serine and threonine are uncharged,they can react within active sites of some enzymes ________.
A)precisely because they are uncharged
B)because the hydroxyl group is polar
C)because the hydroxyl group is small and fits into the site
D)All of the above
A)precisely because they are uncharged
B)because the hydroxyl group is polar
C)because the hydroxyl group is small and fits into the site
D)All of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
33
Cystine is likely to be isolated from proteins that are
A)high in methionine.
B)in the cell nucleus.
C)intracellular.
D)extracellular.
A)high in methionine.
B)in the cell nucleus.
C)intracellular.
D)extracellular.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
34
The pKa's of arginine's α-Carboxyl group,α-Amino group and side chain are 1.8,9.0 and 12.5,respectively.Calculate the isoelectric point.
A)7.8
B)7.2
C)10.8
D)5.4
A)7.8
B)7.2
C)10.8
D)5.4

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
35
An amino acid named for a plant from which it was first isolated is
A)proline.
B)methionine.
C)threonine.
D)asparagine.
A)proline.
B)methionine.
C)threonine.
D)asparagine.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
36
Basic amino acids are ________ (positive,negative)at pH 7 and acidic R group amino acids are ________ (positive,negative)at pH 7.
A)negative;positive
B)negative;negative
C)positive;negative
D)positive;positive
A)negative;positive
B)negative;negative
C)positive;negative
D)positive;positive

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
37
Hydropathy is an important determination of protein-chain folding because
A)it predicts accurately where charged amino acids will appear in a protein.
B)it describes a specific type of folding.
C)it distinguishes enzymes from structural proteins.
D)hydrophobic R groups tend to cluster in the interior of proteins.
A)it predicts accurately where charged amino acids will appear in a protein.
B)it describes a specific type of folding.
C)it distinguishes enzymes from structural proteins.
D)hydrophobic R groups tend to cluster in the interior of proteins.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
38
Histidine has pKa values of 1.8,6.0 (R-group)and 9.3.At pH 8.0,the net charge on histidine is
A)positive.
B)negative.
C)neutral (uncharged).
D)Insufficient information to tell.
A)positive.
B)negative.
C)neutral (uncharged).
D)Insufficient information to tell.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
39
A sequence of amino acids with a relatively high hydropathy is very likely to function by
A)being at the active site of an enzyme.
B)being embedded in a cell membrane.
C)making a protein soluble.
D)being on the protein surface.
A)being at the active site of an enzyme.
B)being embedded in a cell membrane.
C)making a protein soluble.
D)being on the protein surface.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
40
A protein that contains more isoleucine,phenylalanine and leucine than asparagine,lysine and arginine is most likely
A)hydrophilic.
B)hydrophobic.
C)neutral.
D)low on the hydropathy index scale.
A)hydrophilic.
B)hydrophobic.
C)neutral.
D)low on the hydropathy index scale.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
41
The isoelectric point of alanine is pH = 6.15.It is mixed with proline (pHCOOH = 2.0;pHNH₂ = 10.6),and the mixture is placed in an electric field at pH 6.15.Which statement is true?
A)The two amino acids will be separated.
B)The two amino acids will not be separated.
C)Neither amino acid will move in the electric field.
D)Both amino acids will move from the origin and be separated.
A)The two amino acids will be separated.
B)The two amino acids will not be separated.
C)Neither amino acid will move in the electric field.
D)Both amino acids will move from the origin and be separated.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
42
Which shows a proper peptide bond?
A)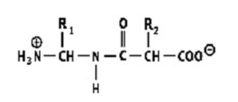
B)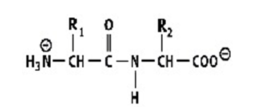
C)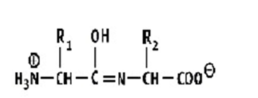
D)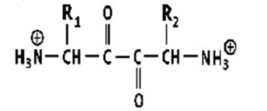
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
43
What is the N-terminal for the pentapeptide Val-Ile-Glu-Arg-Tyr?
A)The NH₃+ group on the side chain of Arg.
B)Valine.
C)Tyrosine.
D)Tryptophan.
A)The NH₃+ group on the side chain of Arg.
B)Valine.
C)Tyrosine.
D)Tryptophan.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
44
In a biochemistry laboratory a student added ammonium sulfate to a tube containing a buffered protein solution.The student then centrifuged the solution.What was the student probably trying to do?
A)Change the pH of the buffer solution to solubilize all proteins.
B)Hydrolyze the proteins into their constituent amino acids to determine the percent composition.
C)Derivatize the N-terminal amino acid of all proteins.
D)Selectively precipitate and purify a certain protein.
A)Change the pH of the buffer solution to solubilize all proteins.
B)Hydrolyze the proteins into their constituent amino acids to determine the percent composition.
C)Derivatize the N-terminal amino acid of all proteins.
D)Selectively precipitate and purify a certain protein.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
45
What is the net charge on the dipeptide Arg-Pro at pH 9.0? The table below gives the pKa's of the ionizable groups on the free amino acids. 
A)-1
B)-0.5
C)0
D)+0.5
E)+1

A)-1
B)-0.5
C)0
D)+0.5
E)+1

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
46
The peptide bond is which of the following?
A)An amide bond.
B)An ester bond.
C)An ether bond.
D)An amine bond.
A)An amide bond.
B)An ester bond.
C)An ether bond.
D)An amine bond.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
47
Ammonium sulfate is often used as a first step in protein purification because it
A)buffers solutions and protects the protein.
B)precipitates many proteins.
C)precipitates all proteins.
D)hydrolyzes proteins into their constituent amino acids.
A)buffers solutions and protects the protein.
B)precipitates many proteins.
C)precipitates all proteins.
D)hydrolyzes proteins into their constituent amino acids.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
48
What is the net charge on the tripeptide Gly-Arg-Lys at pH 7? The table below gives the pKa's of the ionizable groups on the free amino acids. 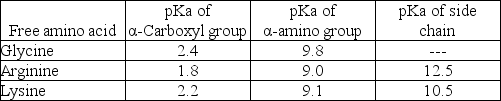
A)-1
B)0
C)+1
D)+2

A)-1
B)0
C)+1
D)+2

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
49
For the amino acid lysine,the Henderson-Hasselbalch equation can be applied to ________ ionization group(s).
A)one
B)two
C)three
D)four
A)one
B)two
C)three
D)four

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
50
The pKa of a certain weak acid is 4.0.Calculate the ratio of proton acceptor to proton donor at pH 7.0.
A)1000:1
B)20:1
C)3:1
D)1:1
E)The ratio cannot be calculated without knowing the structure of the weak acid.
A)1000:1
B)20:1
C)3:1
D)1:1
E)The ratio cannot be calculated without knowing the structure of the weak acid.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
51
The pKa's of the side chain group and the α-carboxyl group of glutamate are 4.1 and 2.1,respectively.Which statement accounts for this difference?
A)The side chain has more possible resonance structures.
B)The α-carboxyl group has less steric hindrance and is therefore ionized more easily.
C)The side chain is a different functional group than the α-carboxyl group.
D)The α-carboxyl group is closer to the α-amino group than the side chain is.
A)The side chain has more possible resonance structures.
B)The α-carboxyl group has less steric hindrance and is therefore ionized more easily.
C)The side chain is a different functional group than the α-carboxyl group.
D)The α-carboxyl group is closer to the α-amino group than the side chain is.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
52
An enzyme works well at as a catalyst at a pH of 7.2.It is found that catalytic activity is significantly less at a pH of 8.4.Which could likely cause the decrease in activity?
A)The protein has been degraded into its amino acids at pH 8.4.
B)The protein has changed shape due to a change in charge.
C)The protonation state of amino acids involved in the catalytic mechanism has changed.
D)B and C above.
E)All of the above.
A)The protein has been degraded into its amino acids at pH 8.4.
B)The protein has changed shape due to a change in charge.
C)The protonation state of amino acids involved in the catalytic mechanism has changed.
D)B and C above.
E)All of the above.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
53
Dialysis during protein purification is a process to
A)concentrate proteins.
B)remove high molecular weight substances.
C)dissolve insoluble proteins.
D)remove low molecular weight solutes such as salts.
A)concentrate proteins.
B)remove high molecular weight substances.
C)dissolve insoluble proteins.
D)remove low molecular weight solutes such as salts.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
54
The first treatment of a crude protein extract usually involves
A)electroplating of unwanted proteins.
B)acidification followed by neutralization.
C)fractionation with varying salt concentrations and centrifugation.
D)treatment with a great number of proteases.
A)electroplating of unwanted proteins.
B)acidification followed by neutralization.
C)fractionation with varying salt concentrations and centrifugation.
D)treatment with a great number of proteases.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
55
Which amino acids are linked in phenylalanylglycine?
A)Phenyline,alanine and glycine.
B)Phenol,alanine and glycine.
C)Phenylalanine and glycine.
D)Phenol,adenine and glycine.
A)Phenyline,alanine and glycine.
B)Phenol,alanine and glycine.
C)Phenylalanine and glycine.
D)Phenol,adenine and glycine.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
56
The ionic charges associated with a protein molecule are ________.
A)mostly contributed by the side chains of constituent amino acids
B)determined by the contribution from the α-carboxyl group,α-amino group and side chain of every amino acid in the protein
C)contributed by only the N-terminal and C-terminal residues
D)independent of the amino acid composition and depend only on pH
A)mostly contributed by the side chains of constituent amino acids
B)determined by the contribution from the α-carboxyl group,α-amino group and side chain of every amino acid in the protein
C)contributed by only the N-terminal and C-terminal residues
D)independent of the amino acid composition and depend only on pH

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
57
How do the pKa values of an ionizable side chain compare when the amino acid is free versus when it is in a polypeptide chain?
A)The pKa of the side chain is independent of whether the amino acid is in a polypeptide chain or is free.
B)The pKa of the side chain is always lower for the free amino acid.
C)The pKa of the side chain may be lower or higher in a polypeptide chain due to weaker inductive effects and differences in their microenvironments.
D)The pKa of the side chain is usually higher in a polypeptide chain due to stabilization from nearby residues in the three-dimensional structure.
A)The pKa of the side chain is independent of whether the amino acid is in a polypeptide chain or is free.
B)The pKa of the side chain is always lower for the free amino acid.
C)The pKa of the side chain may be lower or higher in a polypeptide chain due to weaker inductive effects and differences in their microenvironments.
D)The pKa of the side chain is usually higher in a polypeptide chain due to stabilization from nearby residues in the three-dimensional structure.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
58
Which substance is used to fractionate proteins based on differences in their solubility as a function of salt concentration?
A)Cellophane.
B)Sodium dodecyl sulfate.
C)Phenylisothiocyanate.
D)Ammonium sulfate.
A)Cellophane.
B)Sodium dodecyl sulfate.
C)Phenylisothiocyanate.
D)Ammonium sulfate.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
59
According to the Henderson-Hasselbalch equation,when the concentrations of proton acceptor and proton donor are the same,then
A)the carboxylic acid is totally neutralized.
B)only salt forms are present.
C)pH = pKa.
D)pKa = log[proton acceptor]/[proton donor].
A)the carboxylic acid is totally neutralized.
B)only salt forms are present.
C)pH = pKa.
D)pKa = log[proton acceptor]/[proton donor].

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
60
The primary structure of a protein specifically describes the ________.
A)location of disulfide bonds
B)linear sequence of amino acids
C)overall three-dimensional shape
D)Φ and Ψ angles for each amino acid
A)location of disulfide bonds
B)linear sequence of amino acids
C)overall three-dimensional shape
D)Φ and Ψ angles for each amino acid

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
61
An octapeptide was determined to have the following amino acid composition: Lys (2),Phe (2),Gly (1),His (1),Leu (1),Met (1).The native peptide was run through one cycle of the Edman degradation and the PTH-leucine derivative was identified by HPLC.When the native peptide was exposed to cyanogen bromide (BrCN),a heptapeptide and free glycine were recovered.Incubation of the native protein with trypsin gave a tetrapeptide,a tripeptide,and free lysine.The peptides were separated and each run through one cycle of the Edman degradation.The tetrapeptide yielded the PTH-leucine derivative,and the tripeptide yielded the PTH-phenylalanine derivative.Incubation of the native protein with pepsin produced a dipeptide and two tripeptides.The amino acid composition of the tripeptides (not the order)were determined to be (Phe,Gly,Met)and (Phe,Lys,Lys).What is the sequence of the octapeptide?
Specificities of Proteases
BrCN cuts at the C-terminal side of Met
Trypsin cuts at the C-terminal side of Lys or Arg
Pepsin cuts at the N-terminal side of Phe,Trp or Tyr
A)Leu-His-Phe-Lys-Lys-Phe-Met-Gly
B)Gly-Met-Phe-Lys-Lys-Phe-His-Leu
C)Met-Leu-Phe-Lys-Phe-Gly-Lys-His
D)Leu-His-Lys-Lys-Phe-Phe-Gly-Met
E)His-Phe-Leu-Lys-Lys-Phe-Met-Gly
Specificities of Proteases
BrCN cuts at the C-terminal side of Met
Trypsin cuts at the C-terminal side of Lys or Arg
Pepsin cuts at the N-terminal side of Phe,Trp or Tyr
A)Leu-His-Phe-Lys-Lys-Phe-Met-Gly
B)Gly-Met-Phe-Lys-Lys-Phe-His-Leu
C)Met-Leu-Phe-Lys-Phe-Gly-Lys-His
D)Leu-His-Lys-Lys-Phe-Phe-Gly-Met
E)His-Phe-Leu-Lys-Lys-Phe-Met-Gly

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
62
Which statement is true about SDS-PAGE of proteins?
A)The migration order for proteins in SDS-PAGE is the same as the order in gel filtration because both are sieving techniques.
B)Some proteins migrate toward the anode and others toward the cathode.
C)The rate of migration is inversely proportional to the logarithm of the protein's mass.
D)SDS-PAGE is purely an analytic technique and is not used to purify proteins.
A)The migration order for proteins in SDS-PAGE is the same as the order in gel filtration because both are sieving techniques.
B)Some proteins migrate toward the anode and others toward the cathode.
C)The rate of migration is inversely proportional to the logarithm of the protein's mass.
D)SDS-PAGE is purely an analytic technique and is not used to purify proteins.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
63
Which will react with amino acids to yield derivatives that can be detected by monitoring the absorbance at 254 nm?
A)PITC
B)SDS
C)CNBr
D)HCl
A)PITC
B)SDS
C)CNBr
D)HCl

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
64
The distribution of amino acids in a protein often cannot be determined precisely by acid hydrolysis.Why?
A)The side chains of asparagine and glutamine are also hydrolyzed.
B)The amine groups on lysine and arginine neutralize much of the acid.
C)The side chain of phenylalanine is almost totally destroyed by acid hydrolysis.
D)All of the above.
A)The side chains of asparagine and glutamine are also hydrolyzed.
B)The amine groups on lysine and arginine neutralize much of the acid.
C)The side chain of phenylalanine is almost totally destroyed by acid hydrolysis.
D)All of the above.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
65
The liquid emerging from the bottom of a chromatography column is called the ________.
A)eluate
B)supernatant
C)solute
D)lysate
A)eluate
B)supernatant
C)solute
D)lysate

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
66
Which technique is used less for protein purification and more for the determination of molecular weights?
A)Affinity chromatography.
B)SDS-PAGE.
C)Gel filtration.
D)Ion exchange chromatography.
A)Affinity chromatography.
B)SDS-PAGE.
C)Gel filtration.
D)Ion exchange chromatography.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
67
Which technique is most sensitive and accurate for the determination of a protein's molecular weight?
A)SDS-PAGE.
B)Gel filtration chromatography.
C)Mass spectrometry.
D)X-ray crystallography.
E)Osmotic pressure.
A)SDS-PAGE.
B)Gel filtration chromatography.
C)Mass spectrometry.
D)X-ray crystallography.
E)Osmotic pressure.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
68
In the amino acid analysis the PTC-amino acid derivatives of a hydrolyzed protein are subjected to HPLC.How do you determine which amino acids are present?
A)From their elution time from the HPLC column
B)From the value of the absorbance at 254 nm
C)From the value of the absorbance at 257 nm
D)From conductivity of the separated derivatives
A)From their elution time from the HPLC column
B)From the value of the absorbance at 254 nm
C)From the value of the absorbance at 257 nm
D)From conductivity of the separated derivatives

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
69
What is the purpose of treating a protein with 2-mercaptoethanol?
A)To hydrolyze the protein into its amino acids.
B)To cleave the disulfide bonds.
C)To derivatize any free sulfhydryl groups to prevent them from reforming disulfide bonds.
D)To derivatize the N-terminal amino acid during the Edman degradation.
A)To hydrolyze the protein into its amino acids.
B)To cleave the disulfide bonds.
C)To derivatize any free sulfhydryl groups to prevent them from reforming disulfide bonds.
D)To derivatize the N-terminal amino acid during the Edman degradation.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
70
Even though mass spectrometry has been in use for over a hundred years,it had only limited use with proteins until the 1980s because
A)not many proteins had been discovered and purified before the 1980s.
B)it was not possible to disperse charged proteins into a gaseous stream of particles.
C)many proteins are difficult or impossible to crystallize.
D)proteins decompose too quickly into their component amino acids.
A)not many proteins had been discovered and purified before the 1980s.
B)it was not possible to disperse charged proteins into a gaseous stream of particles.
C)many proteins are difficult or impossible to crystallize.
D)proteins decompose too quickly into their component amino acids.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
71
In which technique is a protein solution pumped through a metal needle at high voltage to create tiny droplets that are analyzed for their mass/charge ratio?
A)Electrospray mass spectrometry.
B)MALDI-TOF.
C)Aerosol microscopy.
D)Millikan analysis.
E)SDS-PAGE.
A)Electrospray mass spectrometry.
B)MALDI-TOF.
C)Aerosol microscopy.
D)Millikan analysis.
E)SDS-PAGE.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
72
What information can be gained by comparing the sequences of proteins that have the same or similar function in different species?
A)To determine evolutionary relationships and relatedness of species.
B)To determine sequences that are conserved among species since they are likely to be important to the function and stability of the proteins.
C)To locate highly variable residues which contribute little to the structure and function of the protein.
D)All of the above.
A)To determine evolutionary relationships and relatedness of species.
B)To determine sequences that are conserved among species since they are likely to be important to the function and stability of the proteins.
C)To locate highly variable residues which contribute little to the structure and function of the protein.
D)All of the above.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
73
A mixture of four proteins (X,Y,Z and N)is applied to a gel-filtration column.Given the information supplied,which will elute first?
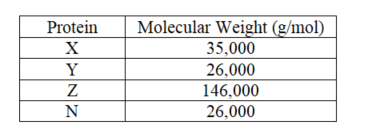
A) Z
B) Y and N elute together
C) X
D) Cannot answer without information about the isoelectric point.

A) Z
B) Y and N elute together
C) X
D) Cannot answer without information about the isoelectric point.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
74
Which is used to hydrolyze the peptide bonds of a protein to yield free amino acids?
A)PITC
B)SDS
C)CNBr
D)HCl
A)PITC
B)SDS
C)CNBr
D)HCl

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
75
Which is used as the basis of separation of proteins by affinity chromatography?
A)The net charge and pI of the protein at the pH of the column.
B)The protein's molecular weight.
C)The protein's density.
D)The selective binding of the protein to a ligand on the column matrix.
A)The net charge and pI of the protein at the pH of the column.
B)The protein's molecular weight.
C)The protein's density.
D)The selective binding of the protein to a ligand on the column matrix.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
76
How does one determine a tryptic fingerprint to identify a protein?
A)Enzymatic treatment followed by mass spectrometry.
B)Enzymatic treatment followed by gel electrophoresis.
C)Treatment with dithiothreitol followed by isoelectric focusing.
D)Treatment with PITC followed by mass spectrometry.
A)Enzymatic treatment followed by mass spectrometry.
B)Enzymatic treatment followed by gel electrophoresis.
C)Treatment with dithiothreitol followed by isoelectric focusing.
D)Treatment with PITC followed by mass spectrometry.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
77
A mixture of two proteins with the same pI and molecular weights (MW)of 10,000 and 15,000 daltons,respectively,are applied to a gel filtration column.What happens during elution?
A)Both elute together.
B)The one with MW of 10,000 elutes first.
C)The one with MW of 15,000 elutes first.
D)More information is needed about the specificity of the column matrix to tell what happens.
A)Both elute together.
B)The one with MW of 10,000 elutes first.
C)The one with MW of 15,000 elutes first.
D)More information is needed about the specificity of the column matrix to tell what happens.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
78
What is the purpose of SDS in SDS-PAGE?
A)To selectively bind the target protein.
B)To maintain buffer pH in the gel.
C)To cause the separation to be on the basis of molecular weight only.
D)To initiate polymerization of acrylamide to form a gel.
A)To selectively bind the target protein.
B)To maintain buffer pH in the gel.
C)To cause the separation to be on the basis of molecular weight only.
D)To initiate polymerization of acrylamide to form a gel.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
79
Gel-filtration chromatography separates a mixture of proteins on the basis of ________.
A)charge
B)size
C)affinity for ligands in the column matrix
D)density
A)charge
B)size
C)affinity for ligands in the column matrix
D)density

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
80
Calculate the approximate relative molecular mass,Mr,for a protein with 527 amino acid residues.
A)5.27 × 106
B)5.8 × 104
C)4.8
D)1.7 × 10-5
E)Cannot determine without knowing the exact sequence of amino acids.
A)5.27 × 106
B)5.8 × 104
C)4.8
D)1.7 × 10-5
E)Cannot determine without knowing the exact sequence of amino acids.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck


