Deck 11: Liquids, Solids, and Intermolecular Forces
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/123
Play
Full screen (f)
Deck 11: Liquids, Solids, and Intermolecular Forces
1
Choose the molecule or compound that exhibits dipole-dipole forces as its strongest intermolecular force.
A)H2
B)SO2
C)NH3
D)CF4
E)BCl3
A)H2
B)SO2
C)NH3
D)CF4
E)BCl3
SO2
2
Choose the pair of substances that are most likely to form a homogeneous solution.
A)NaCl and Hg
B)C3H8 and C2H5OH
C)LiF and C6H14
D)Br2 and PF3
E)NH3 and CH3OH
A)NaCl and Hg
B)C3H8 and C2H5OH
C)LiF and C6H14
D)Br2 and PF3
E)NH3 and CH3OH
NH3 and CH3OH
3
Identify the characteristics of a liquid.
A)indefinite shape and volume
B)indefinite shape,but definite volume
C)definite shape and volume
D)none of the above
E)all of the above
A)indefinite shape and volume
B)indefinite shape,but definite volume
C)definite shape and volume
D)none of the above
E)all of the above
indefinite shape,but definite volume
4
Give the change in condition to go from a liquid to a gas.
A)Increase heat or reduce pressure.
B)Increase heat or increase pressure.
C)Cool or reduce pressure.
D)Cool or increase pressure.
E)None of the above
A)Increase heat or reduce pressure.
B)Increase heat or increase pressure.
C)Cool or reduce pressure.
D)Cool or increase pressure.
E)None of the above

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
5
What type of intermolecular force causes the dissolution of NaCl in water?
A)hydrogen bonding
B)dipole-dipole forces
C)ion-dipole force
D)dispersion forces
E)None of the above
A)hydrogen bonding
B)dipole-dipole forces
C)ion-dipole force
D)dispersion forces
E)None of the above

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
6
Place the following compounds in order of increasing strength of intermolecular forces.
CH4 CH3CH2CH3 CH3CH3
A)CH3CH2CH3 < CH4 < CH3CH3
B)CH3CH2CH3 < CH3CH3 < CH4
C)CH3CH3 < CH4 < CH3CH2CH3
D)CH4 < CH3CH2CH3 < CH3CH3
E)CH4 < CH3CH3 < CH3CH2CH3
CH4 CH3CH2CH3 CH3CH3
A)CH3CH2CH3 < CH4 < CH3CH3
B)CH3CH2CH3 < CH3CH3 < CH4
C)CH3CH3 < CH4 < CH3CH2CH3
D)CH4 < CH3CH2CH3 < CH3CH3
E)CH4 < CH3CH3 < CH3CH2CH3

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
7
Choose the compound that exhibits hydrogen bonding as its strongest intermolecular force.
A)SCl2
B)C2H6
C)CH3OH
D)CH2F2
E)None of the above compounds exhibit hydrogen bonding.
A)SCl2
B)C2H6
C)CH3OH
D)CH2F2
E)None of the above compounds exhibit hydrogen bonding.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
8
Identify the phase in which the water molecules are closest together.
A)gas
B)dry ice
C)solid
D)liquid
A)gas
B)dry ice
C)solid
D)liquid

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
9
What is the strongest type of intermolecular force present in CHF3?
A)ion-dipole
B)dispersion
C)hydrogen bonding
D)dipole-dipole
E)None of the above
A)ion-dipole
B)dispersion
C)hydrogen bonding
D)dipole-dipole
E)None of the above

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
10
Place the following compounds in order of decreasing strength of intermolecular forces.
I.CH3CH2CH2CH2CH2CH3
II.(CH3)3CCH3
III.(CH3)3CCH2CH3
A)III > II > I
B)I > III > II
C)I > II > III
D)II > III > I
E)III > I > II
I.CH3CH2CH2CH2CH2CH3
II.(CH3)3CCH3
III.(CH3)3CCH2CH3
A)III > II > I
B)I > III > II
C)I > II > III
D)II > III > I
E)III > I > II

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
11
Identify the weakest type of intermolecular forces.
A)ion-dipole forces
B)hydrogen bonding
C)dispersion forces
D)dipole-dipole forces
E)ionic forces
A)ion-dipole forces
B)hydrogen bonding
C)dispersion forces
D)dipole-dipole forces
E)ionic forces

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
12
What is the strongest type of intermolecular force present in H2?
A)ion-dipole
B)dipole-dipole
C)dispersion
D)hydrogen bonding
E)None of the above
A)ion-dipole
B)dipole-dipole
C)dispersion
D)hydrogen bonding
E)None of the above

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
13
Choose the molecule or compound that exhibits dispersion forces as its strongest intermolecular force.
A)Cl2
B)CO
C)HF
D)NaCl
E)All of these have intermolecular forces stronger than dispersion.
A)Cl2
B)CO
C)HF
D)NaCl
E)All of these have intermolecular forces stronger than dispersion.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
14
Choose the pair of substances that are most likely to form a homogeneous solution.
A)C6H14 and C10H20
B)LiBr and C5H12
C)N2O4 and NH4Cl
D)C6H14 and H2O
E)None of the pairs above will form a homogeneous solution.
A)C6H14 and C10H20
B)LiBr and C5H12
C)N2O4 and NH4Cl
D)C6H14 and H2O
E)None of the pairs above will form a homogeneous solution.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
15
Place the following compounds in order of increasing strength of intermolecular forces.
CO2 F2 NH2CH3
A)NH2CH3 < CO2 < F2
B)F2 < NH2CH3 < CO2
C)NH2CH3 < F2 < CO2
D)F2 < CO2 < NH2CH3
E)CO2 < NH2CH3 < F2
CO2 F2 NH2CH3
A)NH2CH3 < CO2 < F2
B)F2 < NH2CH3 < CO2
C)NH2CH3 < F2 < CO2
D)F2 < CO2 < NH2CH3
E)CO2 < NH2CH3 < F2

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
16
Choose the pair of substances that are most likely to form a homogeneous solution.
A)CCl4 and SCl2
B)NF3 and SO2
C)CO and C6H6
D)NH2CH3 and CH4
E)None of the pairs above will form a homogeneous solution.
A)CCl4 and SCl2
B)NF3 and SO2
C)CO and C6H6
D)NH2CH3 and CH4
E)None of the pairs above will form a homogeneous solution.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements is true?
A)Intermolecular forces are generally stronger than bonding forces.
B)Until a certain point,the potential energy of molecules decrease as they get closer to one another.
C)Energy is given off when the attraction between two molecules is broken.
D)Increasing the pressure on a solid usually causes it to become a liquid.
E)None of the above are true.
A)Intermolecular forces are generally stronger than bonding forces.
B)Until a certain point,the potential energy of molecules decrease as they get closer to one another.
C)Energy is given off when the attraction between two molecules is broken.
D)Increasing the pressure on a solid usually causes it to become a liquid.
E)None of the above are true.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
18
Place the following compounds in order of decreasing strength of intermolecular forces.
HF O2 CO2
A)HF > CO2 > O2
B)HF > O2 > CO2
C)O2 > CO2 > HF
D)CO2 > HF > O2
E)CO2 > O2 > HF
HF O2 CO2
A)HF > CO2 > O2
B)HF > O2 > CO2
C)O2 > CO2 > HF
D)CO2 > HF > O2
E)CO2 > O2 > HF

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
19
What is the strongest type of intermolecular force present in NH2CH3?
A)dispersion
B)dipole-dipole
C)hydrogen bonding
D)ion-dipole
E)None of the above
A)dispersion
B)dipole-dipole
C)hydrogen bonding
D)ion-dipole
E)None of the above

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
20
Choose the substance with the highest surface tension.
A)HOCH2CH2OH
B)CH2F2
C)CH3CH2F
D)CH3CH2OH
E)CH3CH2CH3
A)HOCH2CH2OH
B)CH2F2
C)CH3CH2F
D)CH3CH2OH
E)CH3CH2CH3

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following statements is true?
A)Vapor pressure increases with temperature.
B)Hydrogen bonds are stronger than covalent bonds.
C)Intermolecular forces hold the atoms in molecules together.
D)Dispersion forces are generally stronger than dipole-dipole forces.
E)None of the above are true.
A)Vapor pressure increases with temperature.
B)Hydrogen bonds are stronger than covalent bonds.
C)Intermolecular forces hold the atoms in molecules together.
D)Dispersion forces are generally stronger than dipole-dipole forces.
E)None of the above are true.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
22
Give the term for the temperature at which the gas and liquid phases form a supercritical fluid.
A)absolute temperature
B)definite temperature
C)fluid temperature
D)critical temperature
E)solid temperature
A)absolute temperature
B)definite temperature
C)fluid temperature
D)critical temperature
E)solid temperature

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
23
Choose the substance with the highest boiling point.
A)CH4
B)KI
C)CS2
D)HF
E)I2
A)CH4
B)KI
C)CS2
D)HF
E)I2

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
24
Choose the substance with the lowest surface tension.
A)CH3SH
B)CH3CH2CH2CH3
C)C6H6
D)H2O
E)(CH3)2CO
A)CH3SH
B)CH3CH2CH2CH3
C)C6H6
D)H2O
E)(CH3)2CO

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
25
How much energy is required to vaporize 48.7 g of dichloromethane (CH2Cl2)at its boiling point,if its ΔHvap is 31.6 kJ/mol?
A)31.2 kJ
B)6.49 kJ
C)55.1 kJ
D)15.4 kJ
E)18.1 kJ
A)31.2 kJ
B)6.49 kJ
C)55.1 kJ
D)15.4 kJ
E)18.1 kJ

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
26
Place the following substances in order of increasing boiling point.
Ne Cl2 O2
A)Ne < Cl2 < O2
B)Cl2 < O2 < Ne
C)O2 < Cl2 < Ne
D)Cl2 < Ne < O2
E)Ne < O2 < Cl2
Ne Cl2 O2
A)Ne < Cl2 < O2
B)Cl2 < O2 < Ne
C)O2 < Cl2 < Ne
D)Cl2 < Ne < O2
E)Ne < O2 < Cl2

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
27
How much energy is required to vaporize 98.6 g of ethanol (C2H5OH)at its boiling point,if its ΔHvap is 40.5 kJ/mol?
A)86.7 kJ
B)11.5 kJ
C)18.9 kJ
D)52.8 kJ
E)39.9 kJ
A)86.7 kJ
B)11.5 kJ
C)18.9 kJ
D)52.8 kJ
E)39.9 kJ

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
28
Place the following substances in order of decreasing vapor pressure at a given temperature.
PF5 BrF3 CF4
A)BrF3 > PF5 > CF4
B)BrF3 > CF4 > PF5
C)PF5 > BrF3 > CF4
D)CF4 > BrF3 > PF5
E)CF4 > PF5 > BrF3
PF5 BrF3 CF4
A)BrF3 > PF5 > CF4
B)BrF3 > CF4 > PF5
C)PF5 > BrF3 > CF4
D)CF4 > BrF3 > PF5
E)CF4 > PF5 > BrF3

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
29
Choose the substance with the lowest boiling point.
A)H2S
B)NBr3
C)F2
D)CF2H2
E)H2O2
A)H2S
B)NBr3
C)F2
D)CF2H2
E)H2O2

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following substances would you predict to have the highest ΔHvap?
A)CH3Cl
B)HCl
C)HOCH2CH2OH
D)CH3CH2OH
E)CH3CH2CH2CH3
A)CH3Cl
B)HCl
C)HOCH2CH2OH
D)CH3CH2OH
E)CH3CH2CH2CH3

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
31
Place the following substances in order of increasing boiling point.
CH3CH2OH Ar CH3OCH3
A)Ar < CH3OCH3 < CH3CH2OH
B)CH3CH2OH < Ar < CH3OCH3
C)CH3CH2OH < CH3OCH3 < Ar
D)CH3OCH3 < Ar < CH3CH2OH
E)Ar < CH3CH2OH < CH3OCH3
CH3CH2OH Ar CH3OCH3
A)Ar < CH3OCH3 < CH3CH2OH
B)CH3CH2OH < Ar < CH3OCH3
C)CH3CH2OH < CH3OCH3 < Ar
D)CH3OCH3 < Ar < CH3CH2OH
E)Ar < CH3CH2OH < CH3OCH3

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
32
Identify the term used to describe the ability of a liquid to flow against gravity up a narrow tube.
A)capillary action
B)viscosity
C)surface tension
D)density
E)None of the above
A)capillary action
B)viscosity
C)surface tension
D)density
E)None of the above

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
33
Choose the substance with the lowest vapor pressure at a given temperature.
A)CO2
B)BeCl2
C)BF3
D)He
E)PF5
A)CO2
B)BeCl2
C)BF3
D)He
E)PF5

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
34
Place the following substances in order of increasing vapor pressure at a given temperature.
SF6 SiH4 SF4
A)SF6 < SiH4 < SF4
B)SiH4 < SF4 < SF6
C)SF6 < SF4 < SiH4
D)SF4 < SF6 < SiH4
E)SiH4 < SF6 < SF4
SF6 SiH4 SF4
A)SF6 < SiH4 < SF4
B)SiH4 < SF4 < SF6
C)SF6 < SF4 < SiH4
D)SF4 < SF6 < SiH4
E)SiH4 < SF6 < SF4

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
35
Choose the substance with the lowest viscosity.
A)Cl3CCCl3
B)Cl2CHCH2Cl
C)Cl2CHCHCl2
D)ClCH2CH2Cl
E)Cl3CCHCl2
A)Cl3CCCl3
B)Cl2CHCH2Cl
C)Cl2CHCHCl2
D)ClCH2CH2Cl
E)Cl3CCHCl2

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
36
How much energy is required to vaporize 158 g of butane (C4H10)at its boiling point,if its ΔHvap is 24.3 kJ/mol?
A)15.1 kJ
B)66.1 kJ
C)89.4 kJ
D)11.2 kJ
E)38.4 kJ
A)15.1 kJ
B)66.1 kJ
C)89.4 kJ
D)11.2 kJ
E)38.4 kJ

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
37
Choose the substance with the highest viscosity.
A)(CH3CH2)2CO
B)C2H4Cl2
C)HOCH2CH2CH2CH2OH
D)CF4
E)C6H14
A)(CH3CH2)2CO
B)C2H4Cl2
C)HOCH2CH2CH2CH2OH
D)CF4
E)C6H14

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
38
Choose the substance with the highest vapor pressure at a given temperature.
A)SiS2
B)RbCl
C)CH3SCH3
D)BF3
E)SbH3
A)SiS2
B)RbCl
C)CH3SCH3
D)BF3
E)SbH3

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following substances would you predict to have the highest ΔHvap?
A)Xe
B)C6H6
C)SiF4
D)Br2
E)N2
A)Xe
B)C6H6
C)SiF4
D)Br2
E)N2

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
40
Which substance below has the strongest intermolecular forces?
A)A2X,ΔHvap = 39.6 kJ/mol
B)BY2,ΔHvap = 26.7 kJ/mol
C)C3X2,ΔHvap = 36.4 kJ/mol
D)DX2,ΔHvap = 23.3 kJ/mol
E)EY3,ΔHvap = 21.5 kJ/mol
A)A2X,ΔHvap = 39.6 kJ/mol
B)BY2,ΔHvap = 26.7 kJ/mol
C)C3X2,ΔHvap = 36.4 kJ/mol
D)DX2,ΔHvap = 23.3 kJ/mol
E)EY3,ΔHvap = 21.5 kJ/mol

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
41
Give the phase transition that occurs as the temperature of dry ice increases.
A)from solid to gas
B)from gas to solid
C)from liquid to gas
D)from liquid to solid
E)from solid to liquid
A)from solid to gas
B)from gas to solid
C)from liquid to gas
D)from liquid to solid
E)from solid to liquid

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
42
Determine ΔHvap for a compound that has a measured vapor pressure of 24.3 torr at 273 K and 135 torr at 325 K.
A)41 kJ/mol
B)79 kJ/mol
C)24 kJ/mol
D)13 kJ/mol
E)34 kJ/mol
A)41 kJ/mol
B)79 kJ/mol
C)24 kJ/mol
D)13 kJ/mol
E)34 kJ/mol

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
43
Determine the normal boiling point of a substance whose vapor pressure is 55.1 mm Hg at 35°C and has a ΔHvap of 32.1 kJ/mol.
A)255 K
B)368 K
C)412 K
D)390.K
E)466 K
A)255 K
B)368 K
C)412 K
D)390.K
E)466 K

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
44
Determine the vapor pressure (in mm Hg)of a substance at 29°C,whose normal boiling point is 76°C and has a ΔHvap of 38.7 kJ/mol.
A)80 mm Hg
B)13 mm Hg
C)21 mm Hg
D)48 mm Hg
E)96 mm Hg
A)80 mm Hg
B)13 mm Hg
C)21 mm Hg
D)48 mm Hg
E)96 mm Hg

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
45
Place the following substances in order of decreasing boiling point.
H2O N2 CO
A)CO > H2O > N2
B)N2 > CO > H2O
C)H2O > CO > N2
D)CO > N2 > H2O
E)N2 > H2O > CO
H2O N2 CO
A)CO > H2O > N2
B)N2 > CO > H2O
C)H2O > CO > N2
D)CO > N2 > H2O
E)N2 > H2O > CO

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
46
Assign the appropriate labels to the phase diagram shown below. 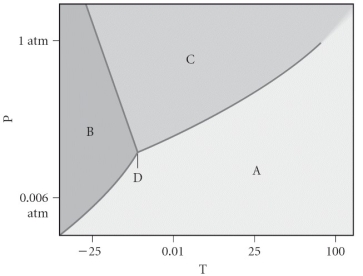
A)A = liquid,B = solid,C = gas,D = critical point
B)A = gas,B = solid,C = liquid,D = triple point
C)A = gas,B = liquid,C = solid,D = critical point
D)A = solid,B = gas,C = liquid,D = supercritical fluid
E)A = liquid,B = gas,C = solid,D = triple point

A)A = liquid,B = solid,C = gas,D = critical point
B)A = gas,B = solid,C = liquid,D = triple point
C)A = gas,B = liquid,C = solid,D = critical point
D)A = solid,B = gas,C = liquid,D = supercritical fluid
E)A = liquid,B = gas,C = solid,D = triple point

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
47
Why is water an extraordinary substance?
A)Water has a low molar mass,yet it is a liquid at room temperature.
B)Water is the main solvent within living organisms.
C)Water has an exceptionally high specific heat capacity.
D)Water has strong hydrogen bonding.
E)All of the above
A)Water has a low molar mass,yet it is a liquid at room temperature.
B)Water is the main solvent within living organisms.
C)Water has an exceptionally high specific heat capacity.
D)Water has strong hydrogen bonding.
E)All of the above

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
48
Consider the phase diagram shown.Choose the statement below that is true. 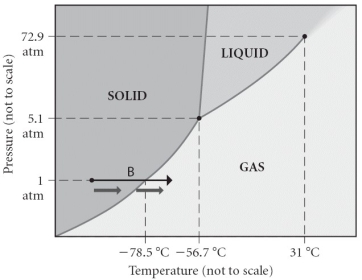
A)The triple point of this substance occurs at a temperature of 31°C.
B)At 10 atm of pressure,there is no temperature where the liquid phase of this substance would exist.
C)The solid phase of this substance is higher in density than the liquid phase.
D)The line separating the solid and liquid phases represents the ΔHvap.
E)None of the above are true.

A)The triple point of this substance occurs at a temperature of 31°C.
B)At 10 atm of pressure,there is no temperature where the liquid phase of this substance would exist.
C)The solid phase of this substance is higher in density than the liquid phase.
D)The line separating the solid and liquid phases represents the ΔHvap.
E)None of the above are true.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
49
Place the following substances in order of decreasing vapor pressure at a given temperature.
BeF2 CH3OH OF2
A)CH3OH > OF2 > BeF2
B)BeF2 > OF2 > CH3OH
C)OF2 > CH3OH > BeF2
D)OF2 > BeF2 > CH3OH
E)BeF2 > CH3OH > OF2
BeF2 CH3OH OF2
A)CH3OH > OF2 > BeF2
B)BeF2 > OF2 > CH3OH
C)OF2 > CH3OH > BeF2
D)OF2 > BeF2 > CH3OH
E)BeF2 > CH3OH > OF2

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
50
How much energy must be removed from a 94.4 g sample of benzene (molar mass = 78.11 g/mol)at 322.0 K to solidify the sample and lower the temperature to 205.0 K? The following physical data may be useful.
ΔHvap = 33.9 kJ/mol
ΔHfus = 9.8 kJ/mol
Cliq = 1.73 J/g°C
Cgas = 1.06 J/g°C
Csol = 1.51 J/g°C
Tmelting = 279.0 K
Tboiling = 353.0 K
A)17.6 kJ
B)11.8 kJ
C)70.2 kJ
D)10.5 kJ
E)29.4 kJ
ΔHvap = 33.9 kJ/mol
ΔHfus = 9.8 kJ/mol
Cliq = 1.73 J/g°C
Cgas = 1.06 J/g°C
Csol = 1.51 J/g°C
Tmelting = 279.0 K
Tboiling = 353.0 K
A)17.6 kJ
B)11.8 kJ
C)70.2 kJ
D)10.5 kJ
E)29.4 kJ

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
51
Define sublimation.
A)The phase transition from solid to gas.
B)The phase transition from gas to solid.
C)The phase transition from gas to liquid.
D)The phase transition from liquid to gas.
E)The phase transition from liquid to solid.
A)The phase transition from solid to gas.
B)The phase transition from gas to solid.
C)The phase transition from gas to liquid.
D)The phase transition from liquid to gas.
E)The phase transition from liquid to solid.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
52
How much energy must be removed from a 125 g sample of benzene (molar mass = 78.11 g/mol)at 425.0 K to liquify the sample and lower the temperature to 335.0 K? The following physical data may be useful.
ΔHvap = 33.9 kJ/mol
ΔHfus = 9.8 kJ/mol
Cliq = 1.73 J/g°C
Cgas = 1.06 J/g°C
Csol = 1.51 J/g°C
Tmelting = 279.0 K
Tboiling = 353.0 K
A)38.9 kJ
B)95.4 kJ
C)67.7 kJ
D)54.3 kJ
E)74.4 kJ
ΔHvap = 33.9 kJ/mol
ΔHfus = 9.8 kJ/mol
Cliq = 1.73 J/g°C
Cgas = 1.06 J/g°C
Csol = 1.51 J/g°C
Tmelting = 279.0 K
Tboiling = 353.0 K
A)38.9 kJ
B)95.4 kJ
C)67.7 kJ
D)54.3 kJ
E)74.4 kJ

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
53
Place the following substances in order of decreasing boiling point.
N2 O2 H2
A)O2 > H2 > N2
B)N2 > H2 > O2
C)N2 > O2 > H2
D)O2 > N2 > H2
E)H2 > N2 > O2
N2 O2 H2
A)O2 > H2 > N2
B)N2 > H2 > O2
C)N2 > O2 > H2
D)O2 > N2 > H2
E)H2 > N2 > O2

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
54
Determine the vapor pressure (in torr)of a substance at 36°C,whose normal boiling point is 84°C and has a ΔHvap of 22.1 kJ/mol.
A)239 torr
B)31.8 torr
C)41.8 torr
D)147 torr
E)98 torr
A)239 torr
B)31.8 torr
C)41.8 torr
D)147 torr
E)98 torr

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
55
Identify triple point.
A)The temperature,pressure,and density for a gas.
B)The temperature at which the boiling point equals the melting point.
C)The temperature and pressure where liquid,solid,and gas are equally stable and are in equilibrium.
D)The temperature that is unique for a substance.
E)The temperature at which the solid and liquid co-exist.
A)The temperature,pressure,and density for a gas.
B)The temperature at which the boiling point equals the melting point.
C)The temperature and pressure where liquid,solid,and gas are equally stable and are in equilibrium.
D)The temperature that is unique for a substance.
E)The temperature at which the solid and liquid co-exist.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
56
How much energy is required to heat 87.1 g acetone (molar mass = 58.08 g/mol)from a solid at -154.0°C to a liquid at -42.0°C? The following physical data may be useful.
ΔHfus = 7.27 kJ/mol
Cliq = 2.16 J/g°C
Cgas = 1.29 J/g°C
Csol = 1.65 J/g°C
Tmelting = -95.0°C
A)8.48 kJ
B)18.5 kJ
C)32.2 kJ
D)29.4 kJ
E)9.97 kJ
ΔHfus = 7.27 kJ/mol
Cliq = 2.16 J/g°C
Cgas = 1.29 J/g°C
Csol = 1.65 J/g°C
Tmelting = -95.0°C
A)8.48 kJ
B)18.5 kJ
C)32.2 kJ
D)29.4 kJ
E)9.97 kJ

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
57
Place the following substances in order of increasing vapor pressure at a given temperature.
NF3 NH3 BCl3
A)NH3 < NF3 < BCl3
B)NF3 < NH3 < BCl3
C)BCl3 < NF3 < NH3
D)NH3 < BCl3 < NF3
E)BCl3 < NH3 < NF3
NF3 NH3 BCl3
A)NH3 < NF3 < BCl3
B)NF3 < NH3 < BCl3
C)BCl3 < NF3 < NH3
D)NH3 < BCl3 < NF3
E)BCl3 < NH3 < NF3

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
58
Consider the phase diagram below.If the dashed line at 1 atm of pressure is followed from 100 to 500°C,what phase changes will occur (in order of increasing temperature)? 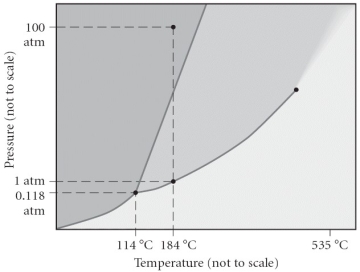
A)condensation,followed by vaporization
B)sublimation,followed by deposition
C)vaporization,followed by deposition
D)fusion,followed by vaporization
E)No phase change will occur under the conditions specified.

A)condensation,followed by vaporization
B)sublimation,followed by deposition
C)vaporization,followed by deposition
D)fusion,followed by vaporization
E)No phase change will occur under the conditions specified.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
59
Define fusion.
A)The phase transition from solid to gas.
B)The phase transition from gas to solid.
C)The phase transition from gas to liquid.
D)The phase transition from liquid to solid.
E)The phase transition from solid to liquid.
A)The phase transition from solid to gas.
B)The phase transition from gas to solid.
C)The phase transition from gas to liquid.
D)The phase transition from liquid to solid.
E)The phase transition from solid to liquid.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
60
How much energy is required to heat 36.0 g H2O from a liquid at 65°C to a gas at 115°C? The following physical data may be useful.
ΔHvap = 40.7 kJ/mol
Cliq = 4.18 J/g°C
Cgas = 2.01 J/g°C
Csol = 2.09 J/g°C
Tmelting = 0°C
Tboiling = 100°C
A)63.5 kJ
B)87.7 kJ
C)10.9 kJ
D)52.7 kJ
E)91.7 kJ
ΔHvap = 40.7 kJ/mol
Cliq = 4.18 J/g°C
Cgas = 2.01 J/g°C
Csol = 2.09 J/g°C
Tmelting = 0°C
Tboiling = 100°C
A)63.5 kJ
B)87.7 kJ
C)10.9 kJ
D)52.7 kJ
E)91.7 kJ

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
61
Explain why the bolling point of water is so much higher than other compounds of similar molecular weight.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
62
Match the following.
CH3CH3
A)dipole-dipole forces
B)dispersion forces
C)ion-dipole forces
D)ionic bond
E)hydrogen bonding
CH3CH3
A)dipole-dipole forces
B)dispersion forces
C)ion-dipole forces
D)ionic bond
E)hydrogen bonding

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
63
Why does the temperature of a substance stay constant during a phase change such as vaporization?

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
64
Vanadium crystallizes in a body-centered cubic structure and has an atomic radius of 131 pm.Determine the density of vanadium,if the edge length of a bcc structure is 4r/ 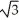
.
A)3.06 g/cm3
B)12.2 g/cm3
C)6.11 g/cm3
D)2.77 g/cm3
E)8.46 g/cm3
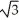
.
A)3.06 g/cm3
B)12.2 g/cm3
C)6.11 g/cm3
D)2.77 g/cm3
E)8.46 g/cm3

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
65
If all of the following are in solid phase,which is considered a non-bonding atomic solid?
A)Ne
B)Fe
C)I2
D)Ca
E)Li
A)Ne
B)Fe
C)I2
D)Ca
E)Li

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
66
Define viscosity.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
67
Match the following.
CH2F2
A)dipole-dipole forces
B)dispersion forces
C)ion-dipole forces
D)ionic bond
E)hydrogen bonding
CH2F2
A)dipole-dipole forces
B)dispersion forces
C)ion-dipole forces
D)ionic bond
E)hydrogen bonding

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following is considered a molecular solid?
A)Cu
B)NH4NO3
C)I2
D)Xe
E)None of these is a molecular solid.
A)Cu
B)NH4NO3
C)I2
D)Xe
E)None of these is a molecular solid.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
69
Match the following.
CH3OH
A)dipole-dipole forces
B)dispersion forces
C)ion-dipole forces
D)ionic bond
E)hydrogen bonding
CH3OH
A)dipole-dipole forces
B)dispersion forces
C)ion-dipole forces
D)ionic bond
E)hydrogen bonding

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
70
Why is the ΔHvap higher than ΔHfus for any given compound?

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
71
Match the following.
LiI
A)dipole-dipole forces
B)dispersion forces
C)ion-dipole forces
D)ionic bond
E)hydrogen bonding
LiI
A)dipole-dipole forces
B)dispersion forces
C)ion-dipole forces
D)ionic bond
E)hydrogen bonding

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following substances should have the highest melting point?
A)CO2
B)SrS
C)Xe
D)F2
E)MgO
A)CO2
B)SrS
C)Xe
D)F2
E)MgO

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
73
A metal crystallizes in a face centered cubic structure and has a density of 11.9 g/cm3.If the radius of the metal atom is 138 pm,what is the identity of the metal?
A)At
B)Pd
C)Mn
D)Fe
E)Cr
A)At
B)Pd
C)Mn
D)Fe
E)Cr

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
74
Determine the radius of an Al atom (in pm)if the density of aluminum is 2.71 g/cm3.Aluminum crystallizes in a face centered cubic structure with an edge length of 2 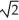
R.
A)143 pm
B)227 pm
C)96 pm
D)172 pm
E)193 pm
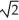
R.
A)143 pm
B)227 pm
C)96 pm
D)172 pm
E)193 pm

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
75
Match the following.
LiI + H2O
A)dipole-dipole forces
B)dispersion forces
C)ion-dipole forces
D)ionic bond
E)hydrogen bonding
LiI + H2O
A)dipole-dipole forces
B)dispersion forces
C)ion-dipole forces
D)ionic bond
E)hydrogen bonding

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
76
Why do O,F and N,when bonded to H,form such strong intermolecular attractions to neighboring molecules? Make sure to be specific.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following is considered an atomic solid?
A)Br2
B)CsCl
C)N2
D)Nb
E)None of these is an atomic solid.
A)Br2
B)CsCl
C)N2
D)Nb
E)None of these is an atomic solid.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
78
Define dynamic equilibrium as it relates to vapor pressure.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following is considered an ionic solid?
A)(NH4)2CO3
B)CCl4
C)SeBr2
D)XeF4
E)None of these is an ionic solid.
A)(NH4)2CO3
B)CCl4
C)SeBr2
D)XeF4
E)None of these is an ionic solid.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following substances should have the highest melting point?
A)Fe
B)Ne
C)Xe
D)N2
E)CO
A)Fe
B)Ne
C)Xe
D)N2
E)CO

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck


