Deck 8: The Nucleus
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/56
Play
Full screen (f)
Deck 8: The Nucleus
1
The mass number of the nucleus 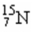 is
is
A)7.
B)8.
C)15.
D)56.
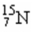 is
isA)7.
B)8.
C)15.
D)56.
15.
2
A particle never emitted in beta decay is the
A)neutron.
B)neutrino.
C)electron.
D)positron.
A)neutron.
B)neutrino.
C)electron.
D)positron.
neutron.
3
The nucleus of a helium atom is called
A)an alpha particle.
B)a beta particle.
C)a gamma ray.
D)a quark.
A)an alpha particle.
B)a beta particle.
C)a gamma ray.
D)a quark.
an alpha particle.
4
Which one or more of the following statements is true in general?
A)Alpha particles are less able to penetrate matter than beta particles.
B)Alpha particles are better able to penetrate matter than beta particles.
C)Alpha particles are less able to penetrate matter than gamma rays.
D)Alpha particles are better able to penetrate matter than gamma rays.
E)Both A and C
A)Alpha particles are less able to penetrate matter than beta particles.
B)Alpha particles are better able to penetrate matter than beta particles.
C)Alpha particles are less able to penetrate matter than gamma rays.
D)Alpha particles are better able to penetrate matter than gamma rays.
E)Both A and C

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
5
A molecule of heavy water contains
A)two oxygen atoms instead of one.
B)three hydrogen atoms instead of two.
C)deuterium atoms instead of hydrogen atoms.
D)a deuterium atom instead of an oxygen atom.
A)two oxygen atoms instead of one.
B)three hydrogen atoms instead of two.
C)deuterium atoms instead of hydrogen atoms.
D)a deuterium atom instead of an oxygen atom.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
6
The electromagnetic waves emitted by a nucleus are called
A)ultraviolet rays.
B)X-rays.
C)gamma rays.
D)quarks.
A)ultraviolet rays.
B)X-rays.
C)gamma rays.
D)quarks.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
7
Of the following particles, the one with the smallest mass is the
A)electron.
B)proton.
C)neutron.
D)neutrino.
A)electron.
B)proton.
C)neutron.
D)neutrino.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
8
The mass of a positron is
A)equal to the mass of an electron.
B)more than the mass of an electron but less than the mass of a proton.
C)equal to the mass of a proton.
D)more than the mass of a proton.
A)equal to the mass of an electron.
B)more than the mass of an electron but less than the mass of a proton.
C)equal to the mass of a proton.
D)more than the mass of a proton.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
9
The number of protons in the nucleus 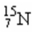 is
is
A)7.
B)8.
C)15.
D)56.
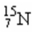 is
isA)7.
B)8.
C)15.
D)56.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
10
A nucleus increases in atomic number when it emits
A)a proton.
B)a neutron.
C)an electron.
D)a positron.
A)a proton.
B)a neutron.
C)an electron.
D)a positron.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
11
The number of protons in a stable nucleus is always
A)less than the number of neutrons.
B)less than or equal to the number of neutrons.
C)equal to or more than the number of neutrons.
D)more than the number of neutrons.
A)less than the number of neutrons.
B)less than or equal to the number of neutrons.
C)equal to or more than the number of neutrons.
D)more than the number of neutrons.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
12
Of the following particles, the one with the greatest mass is the
A)electron.
B)proton.
C)neutron.
D)neutrino.
A)electron.
B)proton.
C)neutron.
D)neutrino.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
13
Which, if any, of the following elements have no radioactive isotopes?
A)hydrogen
B)potassium
C)uranium
D)All of these elements have radioactive isotopes.
A)hydrogen
B)potassium
C)uranium
D)All of these elements have radioactive isotopes.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
14
The number of neutrons in the nucleus 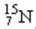 is
is
A)7.
B)8.
C)15.
D)56.
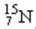 is
isA)7.
B)8.
C)15.
D)56.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
15
The atoms of the isotopes of an element are different in which one or more of the following?
A)number of electrons
B)number of protons
C)number of neutrons
D)atomic mass
E)Both C and D
A)number of electrons
B)number of protons
C)number of neutrons
D)atomic mass
E)Both C and D

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
16
The atomic number of an element determines its
A)mass.
B)binding energy.
C)number of neutrons.
D)chemical behavior.
A)mass.
B)binding energy.
C)number of neutrons.
D)chemical behavior.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
17
The atomic number of the nucleus 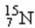 is
is
A)7.
B)8.
C)15.
D)56.
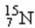 is
isA)7.
B)8.
C)15.
D)56.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
18
The nucleus of an atom cannot be said to
A)contain most of the atom's mass.
B)be small in size.
C)be electrically neutral.
D)deflect alpha particles that come near it.
A)contain most of the atom's mass.
B)be small in size.
C)be electrically neutral.
D)deflect alpha particles that come near it.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is not an isotope of hydrogen?
A)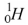
B)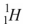
C)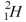
D)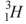
A)
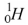
B)
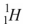
C)
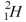
D)
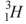

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
20
Of the following particles, the one with the least mass is the
A)electron.
B)proton.
C)neutron.
D)neutrino.
A)electron.
B)proton.
C)neutron.
D)neutrino.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
21
As a sample of a radioactive nuclide decays, its half-life
A)decreases.
B)remains the same.
C)increases.
D)Any of these choices could be correct, depending upon the nuclide.
A)decreases.
B)remains the same.
C)increases.
D)Any of these choices could be correct, depending upon the nuclide.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
22
A reactor does not produce
A)energy.
B)neutrons.
C)uranium.
D)plutonium.
A)energy.
B)neutrons.
C)uranium.
D)plutonium.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
23
The particle that most resembles the proton is the
A)positron.
B)neutron.
C)antiproton.
D)quark.
A)positron.
B)neutron.
C)antiproton.
D)quark.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
24
When a nucleus undergoes radioactive decay, its new mass number is
A)always less than its original mass number.
B)always more than its original mass number.
C)never less than its original mass number.
D)never more than its original mass number.
A)always less than its original mass number.
B)always more than its original mass number.
C)never less than its original mass number.
D)never more than its original mass number.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
25
The radiation dosage due to cosmic rays is greatest
A)in orbiting spacecraft.
B)in airplanes at high altitudes.
C)at sea level.
D)underground.
A)in orbiting spacecraft.
B)in airplanes at high altitudes.
C)at sea level.
D)underground.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
26
Plutonium
A)is an isotope of uranium.
B)is the result of the alpha decay of uranium.
C)is produced by the fission of uranium.
D)has a larger atomic number than uranium.
A)is an isotope of uranium.
B)is the result of the alpha decay of uranium.
C)is produced by the fission of uranium.
D)has a larger atomic number than uranium.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
27
A possible fuel for fusion reactors is
A)ordinary hydrogen.
B)deuterium.
C)uranium.
D)alpha particles.
A)ordinary hydrogen.
B)deuterium.
C)uranium.
D)alpha particles.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
28
A method of compressing fuel for a nuclear fusion reactor that is not being studied uses
A)laser beams.
B)steady magnetic fields.
C)momentary magnetic fields.
D)hydraulic rams.
A)laser beams.
B)steady magnetic fields.
C)momentary magnetic fields.
D)hydraulic rams.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
29
A nucleus with too few neutrons for stability may decay radioactively with the emission of
A)a neutron.
B)a proton.
C)an electron.
D)a positron.
A)a neutron.
B)a proton.
C)an electron.
D)a positron.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
30
The unit in which atomic masses are usually expressed is the
A)gram.
B)eV.
C)u.
D)quark.
A)gram.
B)eV.
C)u.
D)quark.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
31
Nuclear fission can occur in
A)deuterium.
B)hadrons.
C)plutonium.
D)quarks.
A)deuterium.
B)hadrons.
C)plutonium.
D)quarks.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
32
Which one or more of the following is not a unit of energy?
A)joule
B)kilowatt
C)ampere-hour
D)electron volt
E)Both B and C
A)joule
B)kilowatt
C)ampere-hour
D)electron volt
E)Both B and C

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
33
Most known nuclides
A)are stable.
B)are radioactive.
C)can undergo fission.
D)have more nuclear mass than the total mass of their constituent nucleons.
A)are stable.
B)are radioactive.
C)can undergo fission.
D)have more nuclear mass than the total mass of their constituent nucleons.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
34
An atomic nucleus has a mass that is
A)less than the total mass of its constituent nucleons.
B)the same as the total mass of its constituent nucleons.
C)more than the total mass of its constituent nucleons.
D)Any of theses choices could be correct, depending on the nucleus.
A)less than the total mass of its constituent nucleons.
B)the same as the total mass of its constituent nucleons.
C)more than the total mass of its constituent nucleons.
D)Any of theses choices could be correct, depending on the nucleus.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
35
Nuclear fission and fusion reactions give off energy because
A)the binding energy per nucleon is least for nuclei of intermediate size.
B)the binding energy per nucleon is greatest for nuclei of intermediate size.
C)they result in the production of neutrons.
D)they result in the production of plutonium.
A)the binding energy per nucleon is least for nuclei of intermediate size.
B)the binding energy per nucleon is greatest for nuclei of intermediate size.
C)they result in the production of neutrons.
D)they result in the production of plutonium.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
36
In a nuclear power plant, the nuclear reactor itself is used to supply
A)neutrons.
B)electricity.
C)radioactivity.
D)heat.
A)neutrons.
B)electricity.
C)radioactivity.
D)heat.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
37
If radium and chlorine combine to form radium chloride, the compound is
A)no longer radioactive.
B)half as radioactive as its radium content.
C)as radioactive as its radium content.
D)twice as radioactive as its radium content.
A)no longer radioactive.
B)half as radioactive as its radium content.
C)as radioactive as its radium content.
D)twice as radioactive as its radium content.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
38
Slightly enriched uranium can be the energy source of which one or more of the following?
A)nuclear fission reactors
B)nuclear fusion reactors
C)nuclear weapons
D)X-rays
A)nuclear fission reactors
B)nuclear fusion reactors
C)nuclear weapons
D)X-rays

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
39
The process of pair production cannot produce
A)a proton and an antiproton.
B)a neutron and an antineutron.
C)a neutron and a neutrino.
D)an electron and a positron.
A)a proton and an antiproton.
B)a neutron and an antineutron.
C)a neutron and a neutrino.
D)an electron and a positron.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
40
The most important single source of radiation dosage received by an average person in the United States is
A)medical x-rays.
B)nuclear reactors.
C)radon.
D)radium.
A)medical x-rays.
B)nuclear reactors.
C)radon.
D)radium.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
41
All known charged particles have electric charges that are multiples of e except for
A)electrons.
B)positrons.
C)protons.
D)quarks.
A)electrons.
B)positrons.
C)protons.
D)quarks.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
42
The strongest of the fundamental interactions is the
A)gravitational interaction.
B)strong interaction.
C)weak interaction.
D)electromagnetic interaction.
A)gravitational interaction.
B)strong interaction.
C)weak interaction.
D)electromagnetic interaction.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
43
The number of quarks in a proton or a neutron is
A)1.
B)2.
C)3.
D)4.
A)1.
B)2.
C)3.
D)4.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
44
When a nucleus of the copper isotope 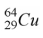 captures an electron, it becomes
captures an electron, it becomes
A)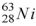 .
.
B)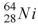 .
.
C)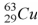 .
.
D)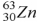 .
.
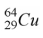 captures an electron, it becomes
captures an electron, it becomesA)
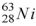 .
.B)
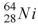 .
.C)
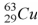 .
.D)
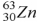 .
.
Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
45
Quarks are not present in
A)electrons.
B)protons.
C)neutrons.
D)hadrons.
A)electrons.
B)protons.
C)neutrons.
D)hadrons.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
46
The half-life of a certain radioactive isotope is 6 h.If we start out with 10 g of the isotope, after 1 day there will be
A)none left.
B)0.625 g left.
C)1.6 g left.
D)2.5 g left.
A)none left.
B)0.625 g left.
C)1.6 g left.
D)2.5 g left.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
47
When a nucleus of the copper isotope 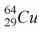 emits a positron, it becomes
emits a positron, it becomes
A)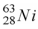 .
.
B)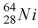 .
.
C)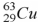 .
.
D)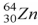 .
.
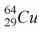 emits a positron, it becomes
emits a positron, it becomesA)
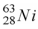 .
.B)
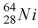 .
.C)
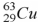 .
.D)
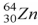 .
.
Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
48
The product of the gamma decay of the strontium isotope 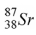 is
is
A)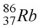 .
.
B)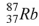 .
.
C)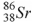 .
.
D)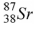 .
.
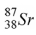 is
isA)
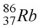 .
.B)
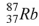 .
.C)
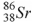 .
.D)
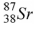 .
.
Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
49
After 10 years, 75 g of an original sample of 100 g of a certain radioactive isotope has decayed.The half-life of the isotope is
A)5 years.
B)7.5 years.
C)20 years.
D)40 years.
A)5 years.
B)7.5 years.
C)20 years.
D)40 years.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
50
The product of the alpha decay of the polonium isotope 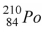 is
is
A)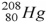 .
.
B)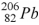 .
.
C)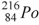 .
.
D)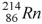 .
.
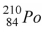 is
isA)
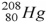 .
.B)
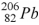 .
.C)
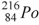 .
.D)
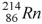 .
.
Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
51
A proton is annihilated when it interacts with which one or more of the following particles?
A)an electron
B)a neutron
C)an antineutron
D)an antiproton
A)an electron
B)a neutron
C)an antineutron
D)an antiproton

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
52
The nuclide that decays by electron capture to become 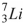 is
is
A)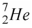 .
.
B)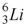 .
.
C)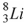 .
.
D)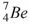 .
.
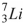 is
isA)
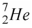 .
.B)
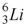 .
.C)
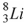 .
.D)
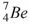 .
.
Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
53
The hydrogen isotope, 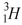 , decays into the helium isotope
, decays into the helium isotope 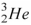 by emitting
by emitting
A)an alpha particle.
B)an electron.
C)a positron.
D)a gamma ray.
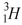 , decays into the helium isotope
, decays into the helium isotope 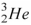 by emitting
by emittingA)an alpha particle.
B)an electron.
C)a positron.
D)a gamma ray.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
54
The helium isotope 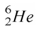 undergoes beta decay with the emission of an electron.The product of the decay is
undergoes beta decay with the emission of an electron.The product of the decay is
A)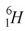 .
.
B)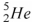 .
.
C)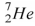 .
.
D)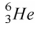 .
.
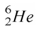 undergoes beta decay with the emission of an electron.The product of the decay is
undergoes beta decay with the emission of an electron.The product of the decay isA)
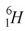 .
.B)
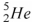 .
.C)
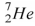 .
.D)
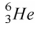 .
.
Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
55
The interaction responsible for the structure of atoms, molecules, liquids, and solids is the
A)gravitational interaction.
B)strong interaction.
C)weak interaction.
D)electromagnetic interaction.
A)gravitational interaction.
B)strong interaction.
C)weak interaction.
D)electromagnetic interaction.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
56
Protons and neutrons are held together to form nuclei by the
A)gravitational interaction.
B)strong interaction.
C)weak interaction.
D)electromagnetic interaction.
A)gravitational interaction.
B)strong interaction.
C)weak interaction.
D)electromagnetic interaction.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck


