Deck 16: Additional Aqueous Equilibria
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/52
Play
Full screen (f)
Deck 16: Additional Aqueous Equilibria
1
In how many different pairs can these substances be mixed to produce buffer solutions? 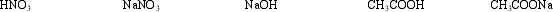
A) 2
B) 3
C) 4
D) 5
E) 6
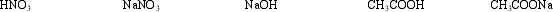
A) 2
B) 3
C) 4
D) 5
E) 6
3
2
A buffer solution is 0.500 M in ascorbic acid and 0.500 M in sodium ascorbate. Its pH is 4.10. After addition of 10 mL of 1 M NaOH to 1.00 L of this buffer, the most likely value of the pH is
A) 4.08.
B) 4.10.
C) 4.12.
D) 5.95.
E) 10.15.
A) 4.08.
B) 4.10.
C) 4.12.
D) 5.95.
E) 10.15.
4.12.
3
An alkaline buffer would best be made using
A) a weak acid with a pKa > 7.00 or Ka < 1.00 × 10-7 and its conjugate base
B) a weak acid with a pKa < 7.00 or Ka > 1.00 × 10-7 and its conjugate base
C) a strong acid and a strong base
D) a weak acid with a Ka > 1.00 × 1014
E) a strong base and its conjugate acid
A) a weak acid with a pKa > 7.00 or Ka < 1.00 × 10-7 and its conjugate base
B) a weak acid with a pKa < 7.00 or Ka > 1.00 × 10-7 and its conjugate base
C) a strong acid and a strong base
D) a weak acid with a Ka > 1.00 × 1014
E) a strong base and its conjugate acid
a weak acid with a pKa > 7.00 or Ka < 1.00 × 10-7 and its conjugate base
4
In a buffer solution, if [A-] = [HA] then
A) pH < pKa
B) pH = pKa
C) pH > pKa
D) pH < 7.00
E) pH > 7.00
A) pH < pKa
B) pH = pKa
C) pH > pKa
D) pH < 7.00
E) pH > 7.00

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
5
A buffer solution is 0.500 M in acetic acid and 0.500 M in sodium acetate. Its pH is 4.74. What is its pH after dilution by a factor of 2?
A) 4.44
B) 4.49
C) 4.74
D) 4.99
E) 5.04
A) 4.44
B) 4.49
C) 4.74
D) 4.99
E) 5.04

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
6
A buffer solution
A) contains more than the expected amount of solute for a particular temperature and is therefore unstable
B) contains the maximum amount of solute possible for a particular temperature
C) changes color upon addition of strong base
D) contains an equal number of hydronium and hydroxide ions
E) resists changes in pH upon addition of acid or base
A) contains more than the expected amount of solute for a particular temperature and is therefore unstable
B) contains the maximum amount of solute possible for a particular temperature
C) changes color upon addition of strong base
D) contains an equal number of hydronium and hydroxide ions
E) resists changes in pH upon addition of acid or base

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
7
Consider a buffer solution made up of H2PO4- and HPO42- For H2PO4-, Ka = 6.2 × 10-8. What mole ratio of HPO42- to H2PO4- will give a pH of 7.35?
A) 0.14 to 1
B) 0.72 to 1
C) 1 to 1
D) 1.4 to 1
E) More information is needed to answer this question.
A) 0.14 to 1
B) 0.72 to 1
C) 1 to 1
D) 1.4 to 1
E) More information is needed to answer this question.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
8
When the concentration of the acid and conjugate base components of a buffer are present in exactly equimolar proportions
A) the buffer is very limited in capacity to absorb either acid or base
B) the buffer has no capacity to absorb any added acid
C) the buffer has no capacity to absorb any added base
D) the buffer has a useful range of pH 6.5 - pH 7.5
E) the pH of the buffer solution is equal to the pKa of the acid component of the buffer
A) the buffer is very limited in capacity to absorb either acid or base
B) the buffer has no capacity to absorb any added acid
C) the buffer has no capacity to absorb any added base
D) the buffer has a useful range of pH 6.5 - pH 7.5
E) the pH of the buffer solution is equal to the pKa of the acid component of the buffer

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
9
In a buffer solution, if [A-] < [HA] then
A) pH < pKa
B) pH = pKa
C) pH > pKa
D) pH < 7.00
E) pH > 7.00
A) pH < pKa
B) pH = pKa
C) pH > pKa
D) pH < 7.00
E) pH > 7.00

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
10
Which statement about buffer solutions is not true?
A) they resist changes in pH upon addition of small quantities of acid or base
B) they consist of approximately equimolar quantities of a weak acid and its conjugate base
C) they consist of approximately equimolar quantities of a weak base and its conjugate acid
D) their acid and base components must react with each other to form new products
E) their pH changes only slightly if the solution is diluted by a factor of 10 with pure water
A) they resist changes in pH upon addition of small quantities of acid or base
B) they consist of approximately equimolar quantities of a weak acid and its conjugate base
C) they consist of approximately equimolar quantities of a weak base and its conjugate acid
D) their acid and base components must react with each other to form new products
E) their pH changes only slightly if the solution is diluted by a factor of 10 with pure water

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
11
A buffer solution is 0.080 M in lactic acid (Ka = 1.8 × 10-4) and 0.070 M in sodium lactate. The pH of the solution is
A) 2.86.
B) 3.68.
C) 3.80.
D) 4.18.
E) 4.62.
A) 2.86.
B) 3.68.
C) 3.80.
D) 4.18.
E) 4.62.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
12
In a buffer solution, if [A-] > [HA], which of the following must be true?
A) pH < pKa
B) pH > pKa
C) pH = pKa
D) pH < 7.00
E) pH > 7.00
A) pH < pKa
B) pH > pKa
C) pH = pKa
D) pH < 7.00
E) pH > 7.00

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
13
A buffer solution will result when ____ is added to a solution of K3PO4.
A) NH3
B) K2HPO4
C) NaOH
D) Na3PO4
E) KCl
A) NH3
B) K2HPO4
C) NaOH
D) Na3PO4
E) KCl

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
14
Which formula represents the Henderson-Hasselbalch equation for the generic acid HA?
A)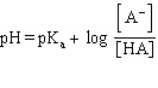
B)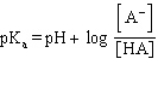
C)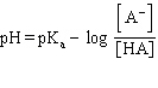
D)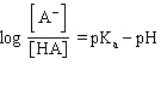
E)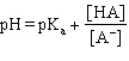
A)
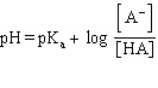
B)
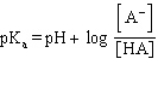
C)
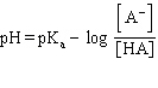
D)
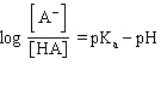
E)
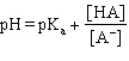

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
15
To make a buffer using acetic acid one could add
A) carbonic acid
B) sodium acetate
C) sodium chloride
D) ammonium chloride
E) ammonium phosphate
A) carbonic acid
B) sodium acetate
C) sodium chloride
D) ammonium chloride
E) ammonium phosphate

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
16
If a buffer is made up using 1.00 mole of a weak acid (pKa = 5.00) and 0.90 mole of its conjugate base, which of the following must be true?
A) pH < 5.00
B) pH = 5.00
C) pH > 5.00
D) pH = 7.00
E) pH > 7.00
A) pH < 5.00
B) pH = 5.00
C) pH > 5.00
D) pH = 7.00
E) pH > 7.00

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
17
Which combination of solutions is the best choice for making a buffer solution?
A) equal volumes of 1 M acetic acid and 0.005 M sodium acetate
B) equal volumes of 0.5 M nitric acid and 0.5 M sodium hydroxide
C) equal volumes of 0.1 M formic acid and 0.1 M sodium formate
D) equal volumes of 0.1 M sulfuric acid and 0.001 M sodium sulfate
E) equal volumes of 0.05 M hydrochloric acid and 0.075 ammonium chloride
A) equal volumes of 1 M acetic acid and 0.005 M sodium acetate
B) equal volumes of 0.5 M nitric acid and 0.5 M sodium hydroxide
C) equal volumes of 0.1 M formic acid and 0.1 M sodium formate
D) equal volumes of 0.1 M sulfuric acid and 0.001 M sodium sulfate
E) equal volumes of 0.05 M hydrochloric acid and 0.075 ammonium chloride

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
18
In order to make up a buffer solution with a neutral pH, it would be best to make use of
A) a weak acid with a Ka at or near 1.00 × 107 and its conjugate base
B) a weak acid with a pKa near 7.00 or a Ka near 1.00 × 10-7 and its conjugate base
C) an equimolar mixture of a strong acid and a strong base
D) an equimolar mixture of an extremely weak acid and an extremely strong base
E) an equimolar mixture of NaCl and HCl
A) a weak acid with a Ka at or near 1.00 × 107 and its conjugate base
B) a weak acid with a pKa near 7.00 or a Ka near 1.00 × 10-7 and its conjugate base
C) an equimolar mixture of a strong acid and a strong base
D) an equimolar mixture of an extremely weak acid and an extremely strong base
E) an equimolar mixture of NaCl and HCl

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
19
Which acid, in combination with its conjugate base, would be the best choice to make a buffer of pH = 4.20?
A) acetic acid (Ka = 1.8 × 10-5)
B) benzoic acid (Ka = 6.3 × 10-5)
C) formic acid (Ka = 1.8 × 10-4)
D) hydrofluoric (Ka = 7.2 × 10-4)
E) nitrous acid (Ka = 4.5 × 10-4)
A) acetic acid (Ka = 1.8 × 10-5)
B) benzoic acid (Ka = 6.3 × 10-5)
C) formic acid (Ka = 1.8 × 10-4)
D) hydrofluoric (Ka = 7.2 × 10-4)
E) nitrous acid (Ka = 4.5 × 10-4)

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
20
What is the smallest amount of solid NaOH shown that will exceed the buffer capacity of 500 mL of a buffer that is 0.40 M in acetic acid and 0.15 M in sodium acetate. (Ka for acetic acid = 1.8 × 10-5.)
A) 8.00 g
B) 5.16 g
C) 4.74 g
D) 4.31 g
E) 3.00 g
A) 8.00 g
B) 5.16 g
C) 4.74 g
D) 4.31 g
E) 3.00 g

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
21
Isotonic saline solution is 0.154 M NaCl(aq). What is the solubility of AgCl (Ksp = 1.8 × 10-10) in such a solution?
A) 2.8 × 10-11 M
B) 1.2 × 10-9 M
C) 5.3 × 10-6 M
D) 3.4 × 10-5 M
E) 0.077 M
A) 2.8 × 10-11 M
B) 1.2 × 10-9 M
C) 5.3 × 10-6 M
D) 3.4 × 10-5 M
E) 0.077 M

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
22
What is the minimum volume of 0.55 M NaOH required to completely convert 1.00 L of 0.45 M acetic acid (pKa = 4.74) to its conjugate base?
A) 0.82 L
B) 1.0 L
C) 1.2 L
D) 1.8 L
E) 2.2 L
A) 0.82 L
B) 1.0 L
C) 1.2 L
D) 1.8 L
E) 2.2 L

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
23
Exhibit 16-1 The following question(s) refer to the same titration: a 50.00 mL sample of 0.0950 M acetic acid (Ka = 1.8 × 10-5) is being titrated with 0.106 M NaOH.
Refer to Exhibit 16-1. What is the pH at the midpoint of the titration?
A) 3.06
B) 3.18
C) 4.44
D) 4.74
E) 5.04
Refer to Exhibit 16-1. What is the pH at the midpoint of the titration?
A) 3.06
B) 3.18
C) 4.44
D) 4.74
E) 5.04

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following indicator(s) would be most suitable for the titration of acetic acid (pKa = 4.74) with NaOH? Your choices are:
I. bromothymol blue (transition range pH 6 to 8)
II. methyl red (transition range pH 4 to 6.3)
III. phenolphthalein (transition range pH 8.3 to 11)
A) I only
B) II only
C) III only
D) I or II only
E) I, II, or III
I. bromothymol blue (transition range pH 6 to 8)
II. methyl red (transition range pH 4 to 6.3)
III. phenolphthalein (transition range pH 8.3 to 11)
A) I only
B) II only
C) III only
D) I or II only
E) I, II, or III

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
25
The Ksp expression for silver phosphate, Ag3PO4, is
A)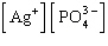
B)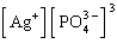
C)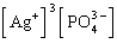
D)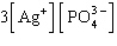
E)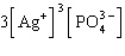
A)
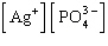
B)
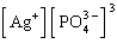
C)
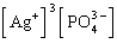
D)
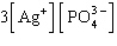
E)
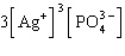

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
26
Which acid-base titration would yield a titration curve of the general form shown? 
A) H2CO3 titrated with NaOH
B) NaOH titrated with H3PO4
C) Na3PO4 titrated with HCl
D) H2SO4 titrated with NaOH
E) H3PO4 titrated with NaOH

A) H2CO3 titrated with NaOH
B) NaOH titrated with H3PO4
C) Na3PO4 titrated with HCl
D) H2SO4 titrated with NaOH
E) H3PO4 titrated with NaOH

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
27
Exhibit 16-1 The following question(s) refer to the same titration: a 50.00 mL sample of 0.0950 M acetic acid (Ka = 1.8 × 10-5) is being titrated with 0.106 M NaOH.
Refer to Exhibit 16-1. What is the pH at the equivalence point of the titration?
A) 5.28
B) 7.00
C) 8.72
D) 9.26
E) Need more information to answer
Refer to Exhibit 16-1. What is the pH at the equivalence point of the titration?
A) 5.28
B) 7.00
C) 8.72
D) 9.26
E) Need more information to answer

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
28
When conducting a titration of an acid by adding base to the equivalence point, it is appropriate to use an indicator which has
A) a pKa = 7.00
B) a color change at a pH close to the pH at the equivalence point
C) a color change at a pH considerably lower than the pH at the equivalence point
D) a color change at a pH considerably higher than the pH at the equivalence point
E) an easily detected color change regardless of the pH where the change occurs
A) a pKa = 7.00
B) a color change at a pH close to the pH at the equivalence point
C) a color change at a pH considerably lower than the pH at the equivalence point
D) a color change at a pH considerably higher than the pH at the equivalence point
E) an easily detected color change regardless of the pH where the change occurs

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
29
What volume of 0.1060 M NaOH is needed to neutralize a 50.00 mL sample of 0.09500 M HNO3?
A) 55.79 mL
B) 55.19 mL
C) 50.00 mL
D) 44.81 mL
E) 5.19 mL
A) 55.79 mL
B) 55.19 mL
C) 50.00 mL
D) 44.81 mL
E) 5.19 mL

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
30
The greater the Ksp of a salt
A) the lower its solubility in water
B) the greater its solubility in water
C) the more easily it is dissolved in all solvents
D) the more easily it dissolves in non-hydrogen bonding solvents
E) the smaller the mass of solute required to prepare a saturated solution
A) the lower its solubility in water
B) the greater its solubility in water
C) the more easily it is dissolved in all solvents
D) the more easily it dissolves in non-hydrogen bonding solvents
E) the smaller the mass of solute required to prepare a saturated solution

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
31
Which statement about the titration of 0.10 M HNO3 with 0.10 M KOH is not correct?
A) The endpoint always comes before the equivalence point.
B) The pH at the equivalence point is 7.00.
C) The initial pH is 1.00.
D) At the equivalence point the pH increases sharply.
E) The net ionic equation for the titration reaction is H3O+ + OH- 2 H2O.
A) The endpoint always comes before the equivalence point.
B) The pH at the equivalence point is 7.00.
C) The initial pH is 1.00.
D) At the equivalence point the pH increases sharply.
E) The net ionic equation for the titration reaction is H3O+ + OH- 2 H2O.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
32
Silver chloride has Ksp = 1.8 × 10-10. What is its molar solubility in water?
A) 9.0 × 10-11 M
B) 3.6 × 10-10 M
C) 6.7 × 10-6 M
D) 9.5 × 10-6 M
E) 1.3 × 10-5 M
A) 9.0 × 10-11 M
B) 3.6 × 10-10 M
C) 6.7 × 10-6 M
D) 9.5 × 10-6 M
E) 1.3 × 10-5 M

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
33
The common ion effect predicts that the solubility of a salt in a solution already containing one of the ions in the salt will be ____ than the solubility in water. This is predictable, because ____.
A) more; Le Chatelier's Principle predicts that the common ion will suppress precipitation
B) more; the presence of the common ion will increase that concentration, hence Ksp will fall
C) less; Le Chatelier's Principle predicts that the common ion will suppress dissolving
D) less; the presence of the common ion will increase that concentration, hence Ksp will rise
E) less; at equilibrium, Q will be less than Ksp
A) more; Le Chatelier's Principle predicts that the common ion will suppress precipitation
B) more; the presence of the common ion will increase that concentration, hence Ksp will fall
C) less; Le Chatelier's Principle predicts that the common ion will suppress dissolving
D) less; the presence of the common ion will increase that concentration, hence Ksp will rise
E) less; at equilibrium, Q will be less than Ksp

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
34
When 1.00 L of 0.45 M acetic acid (pKa = 4.74) is mixed with the exact volume of 0.55 M NaOH required to convert the acid to its conjugate base, at the endpoint the solution will have a
A) pH < 7.00
B) pH > 7.00
C) pH = 7.00
D) pH < pKa
E) pH = pKa
A) pH < 7.00
B) pH > 7.00
C) pH = 7.00
D) pH < pKa
E) pH = pKa

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
35
Which statement is incorrect?
A) water-insoluble salts containing anions that are Bronsted-Lowry bases generally dissolve in solutions of strong bases
B) the solubility of an ionic compound is decreased by the presence of a second solute that provides a common ion
C) amphoteric metal hydroxides are soluble in both highly acidic and highly basic solutions
D) complex ion formation allows the solubilization of many otherwise insoluble metal salts
E) the solubility of water-insoluble salts cannot be affected by the addition of acids or bases to the solution.
A) water-insoluble salts containing anions that are Bronsted-Lowry bases generally dissolve in solutions of strong bases
B) the solubility of an ionic compound is decreased by the presence of a second solute that provides a common ion
C) amphoteric metal hydroxides are soluble in both highly acidic and highly basic solutions
D) complex ion formation allows the solubilization of many otherwise insoluble metal salts
E) the solubility of water-insoluble salts cannot be affected by the addition of acids or bases to the solution.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
36
Silver phosphate is less soluble in Na3PO4 than in water because
A) a common ion displaces the solubility equilibrium towards the undissolved solute.
B) some insoluble compounds are amphoteric.
C) the solubility of most salts increases as temperature increases.
D) the formation of complex ions displaces the solubility equilibrium to the right.
E) the solubility of many salts is affected by the pH of the solution.
A) a common ion displaces the solubility equilibrium towards the undissolved solute.
B) some insoluble compounds are amphoteric.
C) the solubility of most salts increases as temperature increases.
D) the formation of complex ions displaces the solubility equilibrium to the right.
E) the solubility of many salts is affected by the pH of the solution.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
37
A 50.00 mL sample of 0.0950 M acetic acid (Ka = 1.8 × 10-5) is being titrated with 0.0848 M NaOH. What is the pH after 28.00 mL of NaOH has been added?
A) 5.04
B) 4.74
C) 4.44
D) 3.18
E) 3.06
A) 5.04
B) 4.74
C) 4.44
D) 3.18
E) 3.06

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
38
In which liquid or solution would calcium phosphate have the greatest solubility?
A) 0.1 M aqueous sodium phosphate
B) distilled water
C) 0.1 M aqueous calcium nitrate
D) saturated aqueous calcium hydroxide
E) 0.1 M aqueous phosphoric acid
A) 0.1 M aqueous sodium phosphate
B) distilled water
C) 0.1 M aqueous calcium nitrate
D) saturated aqueous calcium hydroxide
E) 0.1 M aqueous phosphoric acid

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
39
The solubility of silver chloride in water is increased by the addition of NH3. Why?
A) The solubility of many salts is affected by the pH of the solution.
B) The formation of complex ions displaces the solubility equilibrium to the right.
C) The solubility of most salts increases as temperature increases.
D) Some insoluble compounds are amphoteric.
E) A common ion displaces the solubility equilibrium toward the undissolved solute.
A) The solubility of many salts is affected by the pH of the solution.
B) The formation of complex ions displaces the solubility equilibrium to the right.
C) The solubility of most salts increases as temperature increases.
D) Some insoluble compounds are amphoteric.
E) A common ion displaces the solubility equilibrium toward the undissolved solute.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
40
Exhibit 16-1 The following question(s) refer to the same titration: a 50.00 mL sample of 0.0950 M acetic acid (Ka = 1.8 × 10-5) is being titrated with 0.106 M NaOH.
Refer to Exhibit 16-1. What is the pH at the endpoint of the titration?
A) 5.28
B) 7.00
C) 8.72
D) 9.26
E) Need more information to answer
Refer to Exhibit 16-1. What is the pH at the endpoint of the titration?
A) 5.28
B) 7.00
C) 8.72
D) 9.26
E) Need more information to answer

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
41
Will a precipitate form when 10.0 mL of 0.500 M NaCl is added to 10.0 mL of 0.0500 M AgNO3? The Ksp for AgCl is 1.8 × 10-10.
A) Yes, because Q < Ksp
B) Yes, because Q = Ksp
C) Yes, because Q > Ksp
D) No, because Q < Ksp
E) No, because Q > Ksp
A) Yes, because Q < Ksp
B) Yes, because Q = Ksp
C) Yes, because Q > Ksp
D) No, because Q < Ksp
E) No, because Q > Ksp

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
42
Answer the following questions:
a. Explain why the solubility of carbonates and phosphates is increased by lowering the pH, but the solubility of chlorides is unaffected by lowering the pH.
b. The solubility of copper(II) carbonate is dramatically increased by the addition of NH3, but the solubility of copper(II) phosphate is not. Referring to the data below, explain why.
Ksp for copper(II) carbonate = 2.5 ´ 10-10
Ksp for copper(II) phosphate = 1.4 ´ 10-37
Cu2+(aq) + 4 NH3(aq)
(Kf = 1.1 ´ 1013)
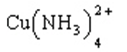
(aq)
a. Explain why the solubility of carbonates and phosphates is increased by lowering the pH, but the solubility of chlorides is unaffected by lowering the pH.
b. The solubility of copper(II) carbonate is dramatically increased by the addition of NH3, but the solubility of copper(II) phosphate is not. Referring to the data below, explain why.
Ksp for copper(II) carbonate = 2.5 ´ 10-10
Ksp for copper(II) phosphate = 1.4 ´ 10-37
Cu2+(aq) + 4 NH3(aq)
(Kf = 1.1 ´ 1013)
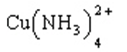
(aq)

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
43
A student attempts to dissolve a small amount of CuCO3 in 0.0100 M NaOH. Despite vigorous stirring, the material does not apparently dissolve. However, after a while the student notices that the solid has changed appearance. When the pH of the solution is measured, it is found to have gone down. Explain these observations. Was the original goal of the experiment achieved?

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
44
In which aqueous solution would the smallest amount of barium sulfate dissolve?
A) 0.1 M Mg(OH)2
B) 0.1 M Na2SO4
C) 0.1 M Al2(SO4)3
D) 0.1 M Ba(NO3)2
E) 0.1 M HCl
A) 0.1 M Mg(OH)2
B) 0.1 M Na2SO4
C) 0.1 M Al2(SO4)3
D) 0.1 M Ba(NO3)2
E) 0.1 M HCl

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
45
A weak acid-strong base titration is being performed using a visual indicator. By titrating a standard acid sample, it is found that the end point is occurring before the equivalence point.
a. Define endpoint and equivalence point.
b. Explain the probable origin of the error, and how you would modify the titration procedure to eliminate it.
a. Define endpoint and equivalence point.
b. Explain the probable origin of the error, and how you would modify the titration procedure to eliminate it.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
46
In which liquid or solution would CuI(s) have the greatest solubility?
A) 0.1 M aqueous ammonia
B) distilled water
C) 0.1 M aqueous copper(I) nitrate
D) saturated aqueous calcium hydroxide
E) 0.1 M aqueous sodium iodide
A) 0.1 M aqueous ammonia
B) distilled water
C) 0.1 M aqueous copper(I) nitrate
D) saturated aqueous calcium hydroxide
E) 0.1 M aqueous sodium iodide

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
47
What is the largest mass of solid NaCl that can be dissolved in 1.000 L of 5.00 × 10-3 M AgNO3 without a precipitate of AgCl forming? Ksp for AgCl is 1.8 × 10-10.
A) 0.78 mg
B) 86 mg
C) 13 mg
D) 2.1 mg
E) 36 ng
A) 0.78 mg
B) 86 mg
C) 13 mg
D) 2.1 mg
E) 36 ng

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
48
If solid sodium chloride is added to a saturated aqueous solution of sodium chloride
A) the total dissolved solids concentration in the solution will increase
B) the anion concentration in the solution will increase
C) the cation concentration in the solution will increase
D) both the anion and anion concentrations in the solution will decrease
E) there will be no change in the anion or cation concentrations in the solution
A) the total dissolved solids concentration in the solution will increase
B) the anion concentration in the solution will increase
C) the cation concentration in the solution will increase
D) both the anion and anion concentrations in the solution will decrease
E) there will be no change in the anion or cation concentrations in the solution

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
49
A solution contains 0.100 M Pb(NO3)2 and 1.00 × 10-5 M AgNO3. It is intended to separate out the silver by selective precipitation of AgI. What is the maximum percentage of the total silver that can be recovered free of contamination by PbI2?
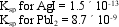
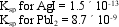

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
50
A mixture of two metal ions in solution is to be separated by selective precipitation. As the concentration of the precipitating ion is increased, which metal ion precipitates first?
A) The one with the lower value of Ksp for its precipitate
B) The one with the higher value of Ksp for its precipitate
C) The one present at higher concentration
D) The one present at lower concentration
E) Cannot answer the question without more information
A) The one with the lower value of Ksp for its precipitate
B) The one with the higher value of Ksp for its precipitate
C) The one present at higher concentration
D) The one present at lower concentration
E) Cannot answer the question without more information

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
51
Which group contains only solutes that would decrease the solubility of barium sulfate?
A) HNO3, H2SO4, HCH3COO
B) SO2, CO2, NH3
C) Ba(NO3)2, Na2SO4, H2SO4
D) Ba(OH)2, NaOH, NH4OH
E) Na2SO4, NaOH, NaCH3COO
A) HNO3, H2SO4, HCH3COO
B) SO2, CO2, NH3
C) Ba(NO3)2, Na2SO4, H2SO4
D) Ba(OH)2, NaOH, NH4OH
E) Na2SO4, NaOH, NaCH3COO

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
52
For a solution containing a dissolved salt, if Q = Ksp
A) the solution is unsaturated and no precipitate forms
B) the solution is unsaturated but is at the point of precipitation
C) the solution is supersaturated and a precipitate forms
D) a precipitate will form from an unsaturated solution
E) the solution is saturated and a precipitate will form if more anions and/or cations are added
A) the solution is unsaturated and no precipitate forms
B) the solution is unsaturated but is at the point of precipitation
C) the solution is supersaturated and a precipitate forms
D) a precipitate will form from an unsaturated solution
E) the solution is saturated and a precipitate will form if more anions and/or cations are added

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck


