Deck 15: Acids and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/62
Play
Full screen (f)
Deck 15: Acids and Bases
1
Carboxylic acids have the formula
A) R-CH3
B) R-CHO
C) R-COOH
D) R-NH2
E) R-OH
A) R-CH3
B) R-CHO
C) R-COOH
D) R-NH2
E) R-OH
R-COOH
2
In terms of acid strength, which acid does not belong in a category with the others?
A) HNO2
B) HClO4
C) HCOOH
D) HF
E) H2SO3
A) HNO2
B) HClO4
C) HCOOH
D) HF
E) H2SO3
HClO4
3
Which reaction illustrates water acting as a base?
A) Cu(H2O)42+ + 4 NH3 Cu(NH3)42+ + 4 H2O
B) H2CO3 H2O + CO2
C) NH3 + H2O NH4+ + OH-
D) H2PO4- + H2O OH- + H3PO4
E) HSO4- + H2O H3O+ + SO42-
A) Cu(H2O)42+ + 4 NH3 Cu(NH3)42+ + 4 H2O
B) H2CO3 H2O + CO2
C) NH3 + H2O NH4+ + OH-
D) H2PO4- + H2O OH- + H3PO4
E) HSO4- + H2O H3O+ + SO42-
HSO4- + H2O H3O+ + SO42-
4
A Bronsted-Lowry base must have a(n) ____.
A) atom that does not obey the octet rule
B) atom with an unshared pair of electrons
C) nitrogen atom
D) amphiprotic atom
E) oxygen atom
A) atom that does not obey the octet rule
B) atom with an unshared pair of electrons
C) nitrogen atom
D) amphiprotic atom
E) oxygen atom

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
5
A Bronsted-Lowry acid is always
A) a highly dissociated acid
B) a weak acid
C) both an acceptor and donor of hydrogen ions
D) a donor of one or more hydrogen ions
E) a molecule or ion in which hydrogen is attached to an oxygen atom
A) a highly dissociated acid
B) a weak acid
C) both an acceptor and donor of hydrogen ions
D) a donor of one or more hydrogen ions
E) a molecule or ion in which hydrogen is attached to an oxygen atom

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
6
Which cannot act as a Bronsted-Lowry acid?
A) HPO42-
B) H3O+
C) CH3COO-
D) CH3NH3+
E) HCOOH
A) HPO42-
B) H3O+
C) CH3COO-
D) CH3NH3+
E) HCOOH

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
7
The conjugate base of NH3 is ____.
A) H2O
B) OH-
C)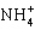
D) NH4OH
E)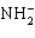
A) H2O
B) OH-
C)
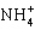
D) NH4OH
E)
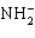

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
8
Bronsted-Lowry acids ____ and Bronsted-Lowry bases ____.
A) generate H3O+ ions in aqueous solution; generate OH- ions in aqueous solutions
B) accept protons; generate OH- ions in aqueous solutions
C) generate OH- ions in aqueous solution; generate H3O+ ions in aqueous solutions
D) generate H3O+ ions in aqueous solution; donate protons in aqueous solutions
E) donate protons; accept protons in aqueous solutions
A) generate H3O+ ions in aqueous solution; generate OH- ions in aqueous solutions
B) accept protons; generate OH- ions in aqueous solutions
C) generate OH- ions in aqueous solution; generate H3O+ ions in aqueous solutions
D) generate H3O+ ions in aqueous solution; donate protons in aqueous solutions
E) donate protons; accept protons in aqueous solutions

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
9
Which pair of substances represents a conjugate acid-base pair?
A) CH3COO- and H2O
B) H3O+ and OH-
C) CH3COOH and CH3COO-
D) CH3COOH and H3O+
E) CH3COOH and OH-
A) CH3COO- and H2O
B) H3O+ and OH-
C) CH3COOH and CH3COO-
D) CH3COOH and H3O+
E) CH3COOH and OH-

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
10
Because water can act as a Bronsted-Lowry acid or base, it is said to be ____.
A) amphiphilic
B) amphihydrous
C) amphiphobic
D) amphiprotic
E) amphoteric
A) amphiphilic
B) amphihydrous
C) amphiphobic
D) amphiprotic
E) amphoteric

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
11
Amines have the general formula
A) R-CHO
B) CH3-(CH2)n-CH3
C) R-COOH
D) R-NH2
E) R-OH
A) R-CHO
B) CH3-(CH2)n-CH3
C) R-COOH
D) R-NH2
E) R-OH

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
12
In this reaction 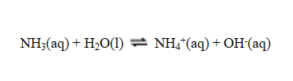
A) NH3 acts as a base and OH- as an acid.
B) H2O acts as an acid and NH3 as a base.
C) H2O acts as a base and NH4+ as an acid.
D) H2O acts as an acid and NH4+ as a base.
E) NH3 acts as an acid and OH- as a base.
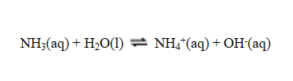
A) NH3 acts as a base and OH- as an acid.
B) H2O acts as an acid and NH3 as a base.
C) H2O acts as a base and NH4+ as an acid.
D) H2O acts as an acid and NH4+ as a base.
E) NH3 acts as an acid and OH- as a base.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
13
In terms of base strength, which base does not belong in a category with the others?
A) Ba(OH)2
B) NaOH
C) Sr(OH)2
D) LiOH
E) NH4OH
A) Ba(OH)2
B) NaOH
C) Sr(OH)2
D) LiOH
E) NH4OH

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
14
Which pair of substances does not represent a conjugate acid-base pair?
A) CH3COOH and CH3COO-
B) CH3NH3+ and CH3NH2
C) H2SO3 and HSO4-
D) HPO42- and PO43-
E) HCOOH and HCOO-
A) CH3COOH and CH3COO-
B) CH3NH3+ and CH3NH2
C) H2SO3 and HSO4-
D) HPO42- and PO43-
E) HCOOH and HCOO-

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
15
The reaction between acetic acid (CH3COOH) and water is written as
A) CH3COOH + H2O CH2COOH- + H3O+
CH2COOH- + H3O+
B) CH3COOH + H2O CH3COO- + OH-
CH3COO- + OH-
C) CH3COOH + H2O CH3COOH2+ + OH-
CH3COOH2+ + OH-
D) CH3COOH + H2O CH3COO- + H3O+
CH3COO- + H3O+
E) CH3COOH + H2O CH3CO- + OH- + H2O
CH3CO- + OH- + H2O
A) CH3COOH + H2O
 CH2COOH- + H3O+
CH2COOH- + H3O+B) CH3COOH + H2O
 CH3COO- + OH-
CH3COO- + OH-C) CH3COOH + H2O
 CH3COOH2+ + OH-
CH3COOH2+ + OH-D) CH3COOH + H2O
 CH3COO- + H3O+
CH3COO- + H3O+E) CH3COOH + H2O
 CH3CO- + OH- + H2O
CH3CO- + OH- + H2O
Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
16
The product of the reaction between methylamine, CH3NH2, and hydrochloric acid are
A) CH4NH2+ and Cl-
B) CH3NH- and H2Cl+
C) CH3NH3+ and Cl-
D) CH3NH3- and Cl+
E) CH3NH42+ and 2Cl-
A) CH4NH2+ and Cl-
B) CH3NH- and H2Cl+
C) CH3NH3+ and Cl-
D) CH3NH3- and Cl+
E) CH3NH42+ and 2Cl-

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
17
Which substance can act as a Bronsted-Lowry base, but not as a Bronsted-Lowry acid?
A) HPO42-
B) H2O
C) NH4+
D) PO43-
E) HSO4-
A) HPO42-
B) H2O
C) NH4+
D) PO43-
E) HSO4-

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
18
Which substance cannot act as a Bronsted-Lowry base?
A) OH-
B) CH3NH2
C) NH4+
D) NH3
E) HSO4-
A) OH-
B) CH3NH2
C) NH4+
D) NH3
E) HSO4-

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
19
Methylamine, CH3NH2, acts as a weak base in water. The products of the reaction are ____ and ____.
A) OH- and CH3NH3+
B) H3O+ and CH3NH3+
C) H3O+ and OH-
D) OH- and CH3NH-
E) H3O+ and CH3NH-
A) OH- and CH3NH3+
B) H3O+ and CH3NH3+
C) H3O+ and OH-
D) OH- and CH3NH-
E) H3O+ and CH3NH-

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
20
Which factor does not contribute to the acidity of the hydrogen atom in the carboxylic acid functional group?
A) the attraction of the O atoms in the carboxylic acid group for the electrons in the O-H bond
B) resonance stabilization of the resulting anion
C) the electronegativity of the C atom
D) the lack of polarity in the C-H bonds in the rest of the molecule
E) the electronegativity of the O that is bonded to the H
A) the attraction of the O atoms in the carboxylic acid group for the electrons in the O-H bond
B) resonance stabilization of the resulting anion
C) the electronegativity of the C atom
D) the lack of polarity in the C-H bonds in the rest of the molecule
E) the electronegativity of the O that is bonded to the H

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
21
One water molecule can donate a proton to another in a process called ____; the equilibrium constant expression for this reaction is ____.
A) neutralization;![<strong>One water molecule can donate a proton to another in a process called ____; the equilibrium constant expression for this reaction is ____.</strong> A) neutralization; B) autoionization; C) protonation; D) hydrolysis; E) autoionization; K<sub>w</sub> = [H<sub>3</sub>O<sup>+</sup>][OH<sup>-</sup>]](https://storage.examlex.com/TB5061/11ea782d_fd7a_dbcb_bbd5_03a8e31609e2_TB5061_11.jpg)
B) autoionization;![<strong>One water molecule can donate a proton to another in a process called ____; the equilibrium constant expression for this reaction is ____.</strong> A) neutralization; B) autoionization; C) protonation; D) hydrolysis; E) autoionization; K<sub>w</sub> = [H<sub>3</sub>O<sup>+</sup>][OH<sup>-</sup>]](https://storage.examlex.com/TB5061/11ea782d_fd7a_dbcc_bbd5_474058719102_TB5061_11.jpg)
C) protonation;![<strong>One water molecule can donate a proton to another in a process called ____; the equilibrium constant expression for this reaction is ____.</strong> A) neutralization; B) autoionization; C) protonation; D) hydrolysis; E) autoionization; K<sub>w</sub> = [H<sub>3</sub>O<sup>+</sup>][OH<sup>-</sup>]](https://storage.examlex.com/TB5061/11ea782d_fd7a_dbcd_bbd5_71a48fb9822a_TB5061_11.jpg)
D) hydrolysis;![<strong>One water molecule can donate a proton to another in a process called ____; the equilibrium constant expression for this reaction is ____.</strong> A) neutralization; B) autoionization; C) protonation; D) hydrolysis; E) autoionization; K<sub>w</sub> = [H<sub>3</sub>O<sup>+</sup>][OH<sup>-</sup>]](https://storage.examlex.com/TB5061/11ea782d_fd7a_dbce_bbd5_699d5bb5f02d_TB5061_11.jpg)
E) autoionization; Kw = [H3O+][OH-]
A) neutralization;
![<strong>One water molecule can donate a proton to another in a process called ____; the equilibrium constant expression for this reaction is ____.</strong> A) neutralization; B) autoionization; C) protonation; D) hydrolysis; E) autoionization; K<sub>w</sub> = [H<sub>3</sub>O<sup>+</sup>][OH<sup>-</sup>]](https://storage.examlex.com/TB5061/11ea782d_fd7a_dbcb_bbd5_03a8e31609e2_TB5061_11.jpg)
B) autoionization;
![<strong>One water molecule can donate a proton to another in a process called ____; the equilibrium constant expression for this reaction is ____.</strong> A) neutralization; B) autoionization; C) protonation; D) hydrolysis; E) autoionization; K<sub>w</sub> = [H<sub>3</sub>O<sup>+</sup>][OH<sup>-</sup>]](https://storage.examlex.com/TB5061/11ea782d_fd7a_dbcc_bbd5_474058719102_TB5061_11.jpg)
C) protonation;
![<strong>One water molecule can donate a proton to another in a process called ____; the equilibrium constant expression for this reaction is ____.</strong> A) neutralization; B) autoionization; C) protonation; D) hydrolysis; E) autoionization; K<sub>w</sub> = [H<sub>3</sub>O<sup>+</sup>][OH<sup>-</sup>]](https://storage.examlex.com/TB5061/11ea782d_fd7a_dbcd_bbd5_71a48fb9822a_TB5061_11.jpg)
D) hydrolysis;
![<strong>One water molecule can donate a proton to another in a process called ____; the equilibrium constant expression for this reaction is ____.</strong> A) neutralization; B) autoionization; C) protonation; D) hydrolysis; E) autoionization; K<sub>w</sub> = [H<sub>3</sub>O<sup>+</sup>][OH<sup>-</sup>]](https://storage.examlex.com/TB5061/11ea782d_fd7a_dbce_bbd5_699d5bb5f02d_TB5061_11.jpg)
E) autoionization; Kw = [H3O+][OH-]

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
22
In an acidic solution at 25 C, which of the following is not true?
A) [H3O+] > 1.0 × 10-7
B) [H3O+] > [OH-]
C) [OH-] < 1.0 × 10-7
D) [H3O+][OH-] > 1.0 × 10-7
E) [H3O+][OH-] < 1.0 × 10-7
A) [H3O+] > 1.0 × 10-7
B) [H3O+] > [OH-]
C) [OH-] < 1.0 × 10-7
D) [H3O+][OH-] > 1.0 × 10-7
E) [H3O+][OH-] < 1.0 × 10-7

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
23
An acidic solution is diluted until [H3O+] is exactly half as much as before. The pH of the solution is now ____ than before.
A) 2.00 higher
B) 0.69 higher
C) 0.30 lower
D) 0.30 higher
E) 2.00 lower
A) 2.00 higher
B) 0.69 higher
C) 0.30 lower
D) 0.30 higher
E) 2.00 lower

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
24
Classify each compound as a carboxylic acid or an amine, in the order given. 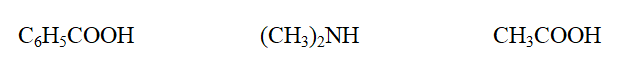
A) acid, acid, acid
B) acid, amine, acid
C) amine, amine, acid
D) amine, acid, amine
E) acid, acid, amine
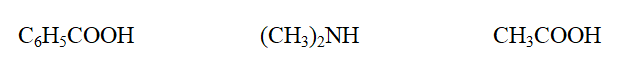
A) acid, acid, acid
B) acid, amine, acid
C) amine, amine, acid
D) amine, acid, amine
E) acid, acid, amine

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
25
Kw for water
A) decreases as the temperature increases
B) increases as the temperature increases
C) is equal to 1.0 × 10-14 at all temperatures
D) is equal to 1.0 × 1014 at all temperatures
E) none of these are correct
A) decreases as the temperature increases
B) increases as the temperature increases
C) is equal to 1.0 × 10-14 at all temperatures
D) is equal to 1.0 × 1014 at all temperatures
E) none of these are correct

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
26
Calculate the pH of a solution where [OH-] = 4.6 × 10-4. Is this solution acidic or basic?
A) 3.34; acidic
B) 3.34; basic
C) 9.40; basic
D) 10.66; acidic
E) 10.66; basic
A) 3.34; acidic
B) 3.34; basic
C) 9.40; basic
D) 10.66; acidic
E) 10.66; basic

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
27
In a 1.2 M solution of HClO4, a strong acid, [H3O+] = ____, and [OH-] = ____.
A) 1.0 × 10-7 M; 1.0 × 10-7 M
B) 8.3 × 10-15 M; 1.0 × 10-14 M
C) 8.3 × 10-15 M; 1.2 M
D) 1.2 M; 8.3 × 10-15 M
E) 1.2 M; 1.2 M
A) 1.0 × 10-7 M; 1.0 × 10-7 M
B) 8.3 × 10-15 M; 1.0 × 10-14 M
C) 8.3 × 10-15 M; 1.2 M
D) 1.2 M; 8.3 × 10-15 M
E) 1.2 M; 1.2 M

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
28
The acid ionization constant expression for the ionization of the hydrogen sulfate ion, 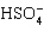 , in aqueous solution is
, in aqueous solution is
A)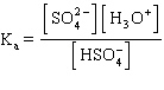
B)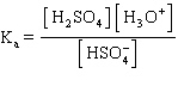
C)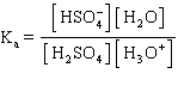
D)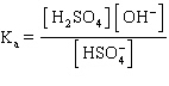
E)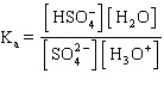
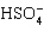 , in aqueous solution is
, in aqueous solution isA)
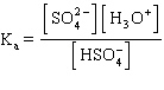
B)
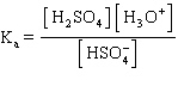
C)
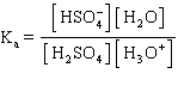
D)
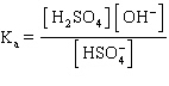
E)
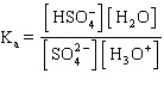

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
29
The larger the value of Ka
A) the less highly dissociated the acid and the weaker the acid
B) the less highly dissociated the acid and the stronger the acid
C) the more highly dissociated the acid and the weaker the acid
D) the more highly dissociated the acid and the stronger the acid
E) the answer depends on whether the acid is strong or weak
A) the less highly dissociated the acid and the weaker the acid
B) the less highly dissociated the acid and the stronger the acid
C) the more highly dissociated the acid and the weaker the acid
D) the more highly dissociated the acid and the stronger the acid
E) the answer depends on whether the acid is strong or weak

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
30
In a basic solution at 25 C,
A) [H3O+] > 1.0 × 10-7.
B) [H3O+] > [OH-].
C) [OH-] > 1.0 × 10-7.
D) [H3O+][OH-] > 1.0 × 10-14.
E) [H3O+][OH-] < 1.0 × 10-14.
A) [H3O+] > 1.0 × 10-7.
B) [H3O+] > [OH-].
C) [OH-] > 1.0 × 10-7.
D) [H3O+][OH-] > 1.0 × 10-14.
E) [H3O+][OH-] < 1.0 × 10-14.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
31
A wine sample had a pH of 3.52. This corresponds to [H3O+] =
A) 3.3 × 10-11 M.
B) 3.0 × 10-4 M.
C) 5.2 × 10-4 M.
D) 5.2 × 10-3 M.
E) 3.3 × 10-3 M.
A) 3.3 × 10-11 M.
B) 3.0 × 10-4 M.
C) 5.2 × 10-4 M.
D) 5.2 × 10-3 M.
E) 3.3 × 10-3 M.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
32
As the pH of a solution rises, [H3O+] ____, pOH ____, and [OH-] ____.
A) rises; falls; rises
B) falls; rises; falls
C) falls; falls; rises
D) rises; falls; falls
E) falls, falls, falls
A) rises; falls; rises
B) falls; rises; falls
C) falls; falls; rises
D) rises; falls; falls
E) falls, falls, falls

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
33
If the pH of a solution is raised from 6.27 to 7.57
A) the solution has gone from acidic to basic
B) [H3O+] has increased by a factor of 10
C) [OH-] has increased by a factor of 10
D) the new [OH-] = 2.7 × 10-8
E) the new [H3O+] = 5.4 × 10-7
A) the solution has gone from acidic to basic
B) [H3O+] has increased by a factor of 10
C) [OH-] has increased by a factor of 10
D) the new [OH-] = 2.7 × 10-8
E) the new [H3O+] = 5.4 × 10-7

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
34
A solution is not neutral. Which one of these statements is true?
A) [H3O+] = 1.0 × 10-7 M
B) [H3O+] = [OH-]
C) [H3O+][OH-] = 1.0 × 10-7
D) [OH-] = 1.0 × 10-7 M
E) [H3O+][OH-] = 1.0 × 10-14
A) [H3O+] = 1.0 × 10-7 M
B) [H3O+] = [OH-]
C) [H3O+][OH-] = 1.0 × 10-7
D) [OH-] = 1.0 × 10-7 M
E) [H3O+][OH-] = 1.0 × 10-14

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
35
In a 1.2 M solution of KOH, a strong base, [H3O+] = ____, and [OH-] = ____.
A) 1.0 × 10-7 M; 1.0 × 10-7 M
B) 8.3 × 10-15 M; 1.0 × 10-14 M
C) 8.3 × 10-15 M; 1.2 M
D) 1.2 M; 8.3 × 10-15 M
E) 1.2 M; 1.2 M
A) 1.0 × 10-7 M; 1.0 × 10-7 M
B) 8.3 × 10-15 M; 1.0 × 10-14 M
C) 8.3 × 10-15 M; 1.2 M
D) 1.2 M; 8.3 × 10-15 M
E) 1.2 M; 1.2 M

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
36
Phenolphthalein is an acid-base indicator that is colorless in its acid form and pink in its basic form, changing at pH = 8.5. Bromcresol green is yellow in its acidic form and blue in its basic form, changing at pH = 4.8. A solution is colorless in phenolphthalein and blue in bromcresol green. Therefore we can conclude that the pH of the solution is ____.
A) exactly 7.0
B) greater than 8.5
C) between 7.0 and 8.5
D) between 4.8 and 8.5
E) less than 4.8
A) exactly 7.0
B) greater than 8.5
C) between 7.0 and 8.5
D) between 4.8 and 8.5
E) less than 4.8

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
37
For the dissociation of a very weak acid, HA, in water
A) Ka = [HA] [H3O+] [A-]
B) Ka = [H3O+] [A-]
C) Ka = [H3O+] [A-] / [H2O]
D) Ka = [HA] / [A-] [H3O+]
E) Ka = [H3O+] [A-] / [HA]
A) Ka = [HA] [H3O+] [A-]
B) Ka = [H3O+] [A-]
C) Ka = [H3O+] [A-] / [H2O]
D) Ka = [HA] / [A-] [H3O+]
E) Ka = [H3O+] [A-] / [HA]

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
38
Arrange the solutions in order of increasing acidity: 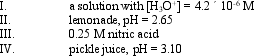
A) I < IV < II < III
B) II < IV < III < I
C) III < II < IV < I
D) IV < I < II < III
E) III < II < I < IV
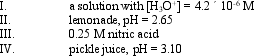
A) I < IV < II < III
B) II < IV < III < I
C) III < II < IV < I
D) IV < I < II < III
E) III < II < I < IV

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
39
Which condition represents the most acidic solution?
A) [H3O+] = .010 M
B) [OH-] = 1.0 × 10-13 M
C) pH = 3.6
D) [OH-] = 1.0 × 10-10 M
E) pOH = 8.3
A) [H3O+] = .010 M
B) [OH-] = 1.0 × 10-13 M
C) pH = 3.6
D) [OH-] = 1.0 × 10-10 M
E) pOH = 8.3

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
40
If the hydrogen ion concentration of a solution is increased by a factor of 4, the pH is
A) raised by 4 pH units
B) raised by 0.3 pH units
C) lowered by 2 pH units
D) lowered by 0.6 pH units
E) lowered by 0.25 pH units
A) raised by 4 pH units
B) raised by 0.3 pH units
C) lowered by 2 pH units
D) lowered by 0.6 pH units
E) lowered by 0.25 pH units

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
41
Ammonia is a weak base and acetic acid is a weak acid. Which statement is true of a solution of ammonium acetate?
A) It is strongly acidic
B) It is weakly acidic
C) It is neutral
D) It is weakly basic
E) We cannot predict its acid-base properties without more information
A) It is strongly acidic
B) It is weakly acidic
C) It is neutral
D) It is weakly basic
E) We cannot predict its acid-base properties without more information

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
42
Calculate the pH of a 0.051 M solution of sodium lactate. The Ka for lactic acid is 1.4 × 10-4.
A) 1.29
B) 2.57
C) 8.28
D) 11.43
E) 12.71
A) 1.29
B) 2.57
C) 8.28
D) 11.43
E) 12.71

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
43
The reaction between the ion of a weak acid or a weak base and water is called a(n) ____ reaction.
A) autoionization
B) hydrolysis
C) decomposition
D) neutralization
E) proteomic
A) autoionization
B) hydrolysis
C) decomposition
D) neutralization
E) proteomic

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
44
Two common polyprotic acids are
A) sulfuric acid and perchloric acid
B) sulfuric acid and nitric acid
C) sulfuric acid and phosphoric acid
D) perchloric acid and nitric acid
E) nitric acid and phosphoric acid
A) sulfuric acid and perchloric acid
B) sulfuric acid and nitric acid
C) sulfuric acid and phosphoric acid
D) perchloric acid and nitric acid
E) nitric acid and phosphoric acid

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
45
The value of the ionization constant for a weak acid HA is 4.2 × 10-7. What is the pH of a 0.35 M solution of this acid?
A) 6.83
B) 6.38
C) 3.42
D) 3.19
E) 2.96
A) 6.83
B) 6.38
C) 3.42
D) 3.19
E) 2.96

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
46
Which salt forms a neutral solution when dissolved in water?
A) LiF
B) K3PO4
C) NH4ClO4
D) NaOCl
E) NaNO3
A) LiF
B) K3PO4
C) NH4ClO4
D) NaOCl
E) NaNO3

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
47
Consider a 0.50 M solution of HNO2, a weak acid with Ka = 4.5 × 10-4. Which statement is true?
A) [H3O+] = 0.50 M.
B) The acid is mostly ionized.
C) pH = 3.35.
D) pH = 0.32.
E) pH > 0.32.
A) [H3O+] = 0.50 M.
B) The acid is mostly ionized.
C) pH = 3.35.
D) pH = 0.32.
E) pH > 0.32.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
48
In a neutral polyprotic acid, the value of Ka2 will be ____ the value of Ka1 because ____.
A) smaller than; it is more difficult to remove a hydrogen ion from a cation than from a neutral molecule
B) larger than; it is more difficult to remove a hydrogen ion from an anion than from a neutral molecule
C) larger than; it is more difficult to remove a hydrogen ion from a cation than from a neutral molecule
D) smaller than; it is more difficult to remove a hydrogen ion from an anion than from a neutral molecule
E) the same as; both hydrogen ions are bonded to the same anion
A) smaller than; it is more difficult to remove a hydrogen ion from a cation than from a neutral molecule
B) larger than; it is more difficult to remove a hydrogen ion from an anion than from a neutral molecule
C) larger than; it is more difficult to remove a hydrogen ion from a cation than from a neutral molecule
D) smaller than; it is more difficult to remove a hydrogen ion from an anion than from a neutral molecule
E) the same as; both hydrogen ions are bonded to the same anion

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
49
Typically, for a weak acid and its conjugate base
A) Ka / Kb = 1.0 × 10-14
B) Ka × Kb = 1.0 × 10-14
C) Ka / Kb = 1.0
D) Ka × Kb = 1.0
E) Ka × Kb = 14
A) Ka / Kb = 1.0 × 10-14
B) Ka × Kb = 1.0 × 10-14
C) Ka / Kb = 1.0
D) Ka × Kb = 1.0
E) Ka × Kb = 14

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
50
Which compound is the most acidic?
A) GeH4
B) H2Se
C) HBr
D) AsH3
E) H2O
A) GeH4
B) H2Se
C) HBr
D) AsH3
E) H2O

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
51
Which substance is a Lewis acid but not a Bronsted-Lowry acid?
A) acetic acid
B) NH4+(aq)
C) HCO3-(aq)
D) CO2
E) CO32-
A) acetic acid
B) NH4+(aq)
C) HCO3-(aq)
D) CO2
E) CO32-

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
52
The poison strychnine is a weakly basic compound with Kb = 1.8 × 10-6. What is the pH of a 0.058 M solution of strychnine?
A) 3.49
B) 7.30
C) 8.26
D) 10.51
E) 12.76
A) 3.49
B) 7.30
C) 8.26
D) 10.51
E) 12.76

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following salts forms a basic solution when dissolved in water?
A) NH4ClO4
B) (NH4)2NO3
C) NaClO4
D) LiCH3COO
E) NaCl
A) NH4ClO4
B) (NH4)2NO3
C) NaClO4
D) LiCH3COO
E) NaCl

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
54
The pH of a solution of a 0.15 M solution of HOCl is 4.14. What is the Ka for HOCl?
A) 5.7 × 10-2
B) 8.8 × 10-3
C) 4.8 × 10-4
D) 7.2 × 10-5
E) 3.5 × 10-8
A) 5.7 × 10-2
B) 8.8 × 10-3
C) 4.8 × 10-4
D) 7.2 × 10-5
E) 3.5 × 10-8

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
55
Ammonia is a weak base and perchloric acid is a strong acid. Which statement is true of a solution of ammonium perchlorate?
A) It is strongly acidic
B) It is weakly acidic
C) It is neutral
D) It is weakly basic
E) We cannot predict its acid-base properties without more information
A) It is strongly acidic
B) It is weakly acidic
C) It is neutral
D) It is weakly basic
E) We cannot predict its acid-base properties without more information

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following salts forms an acidic solution when dissolved in water?
A) LiF
B) K3PO4
C) NH4ClO4
D) NaOCl
E) NaNO3
A) LiF
B) K3PO4
C) NH4ClO4
D) NaOCl
E) NaNO3

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
57
The pH of a 0.50 M solution of the weak monoprotic acid HA is 4.76. The value of Ka is
A) 6.0 × 10-10
B) 1.7 × 10-5
C) 3.5 × 10-5
D) 7.6 × 10-4
E) 9.24
A) 6.0 × 10-10
B) 1.7 × 10-5
C) 3.5 × 10-5
D) 7.6 × 10-4
E) 9.24

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
58
Which statement is not correct?
A) a Lewis acid is a substance that can accept a pair of electrons to form a new bond
B) a Lewis base is a substance that can donate a pair of electrons to form a new bond
C) Al(OH)3 is an amphoteric substance that can form either a positive or negative ion
D) Neutral molecules cannot act as Lewis acids
E) Ag(NH3)2+ is a complex ion, formed from a Ag+ ion (Lewis acid) and two NH3 molecules (Lewis bases)
A) a Lewis acid is a substance that can accept a pair of electrons to form a new bond
B) a Lewis base is a substance that can donate a pair of electrons to form a new bond
C) Al(OH)3 is an amphoteric substance that can form either a positive or negative ion
D) Neutral molecules cannot act as Lewis acids
E) Ag(NH3)2+ is a complex ion, formed from a Ag+ ion (Lewis acid) and two NH3 molecules (Lewis bases)

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
59
The pH of a 0.172 M solution of benzoic acid (Ka = 6.31 × 10-5) is
A) 2.48.
B) 3.44.
C) 4.37.
D) 4.96.
E) 5.63.
A) 2.48.
B) 3.44.
C) 4.37.
D) 4.96.
E) 5.63.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
60
Identify which of the hydrohalic acids is unlike the others, and why.
A) HF(aq), because it is the only strong acid
B) HCl(aq), because it is the only weak acid
C) HBr(aq), because it is a liquid at room temperature
D) HI(aq), because it does not display hydrogen bonding
E) HF(aq), because it displays hydrogen bonding
A) HF(aq), because it is the only strong acid
B) HCl(aq), because it is the only weak acid
C) HBr(aq), because it is a liquid at room temperature
D) HI(aq), because it does not display hydrogen bonding
E) HF(aq), because it displays hydrogen bonding

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
61
Answer the following questions:
A) Write the reaction for the ionization of the weak acid HA in water.
B) Write the reaction for the hydrolysis of the conjugate base of the weak acid HA in water.
C) Using equilibrium constant expressions for the reaction of HA and of its conjugate base A- with water, prove that the product of Ka for a weak acid and Kb for its conjugate base equals 1.0 ´ 10-14 at 25°C.
A) Write the reaction for the ionization of the weak acid HA in water.
B) Write the reaction for the hydrolysis of the conjugate base of the weak acid HA in water.
C) Using equilibrium constant expressions for the reaction of HA and of its conjugate base A- with water, prove that the product of Ka for a weak acid and Kb for its conjugate base equals 1.0 ´ 10-14 at 25°C.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
62
A solution of acetic acid (Ka = 1.8 × 10-5) has a pH exactly 2 higher than a solution of HCl(aq) of the same concentration. What is that concentration?

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck


