Deck 11: Liquids, Solids, and Materials
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/46
Play
Full screen (f)
Deck 11: Liquids, Solids, and Materials
1
What is the correct order for the surface tension of the following substances?
A) CHCl3 < H2O < C8H18
B) H2O < CHCl3 < C8H18
C) CHCl3 < C8H18 < H2O
D) C8H18 < CHCl3 < H2O
E) C8H18 < H2O < CHCl3
A) CHCl3 < H2O < C8H18
B) H2O < CHCl3 < C8H18
C) CHCl3 < C8H18 < H2O
D) C8H18 < CHCl3 < H2O
E) C8H18 < H2O < CHCl3
C8H18 < CHCl3 < H2O
2
Exhibit 11-1 For the following question(s), consider the graphic below: 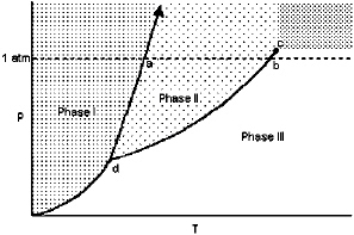 Refer to Exhibit 11-1. The temperature at point a is the
Refer to Exhibit 11-1. The temperature at point a is the
A) critical point.
B) triple point.
C) absolute freezing point.
D) normal freezing point.
E) normal boiling point.
 Refer to Exhibit 11-1. The temperature at point a is the
Refer to Exhibit 11-1. The temperature at point a is theA) critical point.
B) triple point.
C) absolute freezing point.
D) normal freezing point.
E) normal boiling point.
normal freezing point.
3
Volatility is the
A) temperature at which the equilibrium vapor pressure of a liquid is exactly equal to the atmospheric pressure.
B) odor of a substance.
C) temperature at which a gas can be converted into a liquid at atmospheric pressure.
D) tendency of molecules of a gas to move closer together when the pressure is increased.
E) tendency of a liquid to vaporize.
A) temperature at which the equilibrium vapor pressure of a liquid is exactly equal to the atmospheric pressure.
B) odor of a substance.
C) temperature at which a gas can be converted into a liquid at atmospheric pressure.
D) tendency of molecules of a gas to move closer together when the pressure is increased.
E) tendency of a liquid to vaporize.
tendency of a liquid to vaporize.
4
Which statement about relative humidity is false?
A) ratio of actual partial pressure of water to its equilibrium partial pressure
B) rises when the temperature increases and the actual partial pressure of water is constant
C) reaches 100% at the dew point
D) indicates the level of water vapor in the atmosphere
E) the actual partial pressure of water can equal the equilibrium partial pressure
A) ratio of actual partial pressure of water to its equilibrium partial pressure
B) rises when the temperature increases and the actual partial pressure of water is constant
C) reaches 100% at the dew point
D) indicates the level of water vapor in the atmosphere
E) the actual partial pressure of water can equal the equilibrium partial pressure

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
5
Exhibit 11-1 For the following question(s), consider the graphic below: 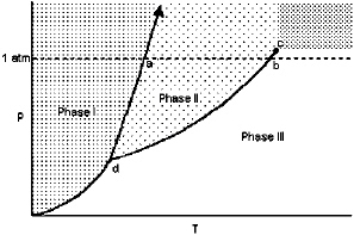 Refer to Exhibit 11-1. The temperature at point d is the
Refer to Exhibit 11-1. The temperature at point d is the
A) critical point.
B) triple point.
C) absolute freezing point.
D) normal freezing point.
E) normal boiling point.
 Refer to Exhibit 11-1. The temperature at point d is the
Refer to Exhibit 11-1. The temperature at point d is theA) critical point.
B) triple point.
C) absolute freezing point.
D) normal freezing point.
E) normal boiling point.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
6
Boiling a liquid means
A) the vapor pressure of the liquid equals the pressure exerted by its vapor phase.
B) a dynamic equilibrium exists between the liquid and vapor phase.
C) the vapor pressure of the liquid equals the external pressure above the liquid surface.
D) the molecular bonds in the liquid are broken rapidly.
E) the vapor pressure of the liquid is less than the external pressure above the liquid surface.
A) the vapor pressure of the liquid equals the pressure exerted by its vapor phase.
B) a dynamic equilibrium exists between the liquid and vapor phase.
C) the vapor pressure of the liquid equals the external pressure above the liquid surface.
D) the molecular bonds in the liquid are broken rapidly.
E) the vapor pressure of the liquid is less than the external pressure above the liquid surface.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
7
The best explanation for the existence of a meniscus observed when water is placed in a glass tube of small diameter is
A) the attractive forces between the water molecules and the walls of the container are greater than the attractive forces between the water molecules.
B) the hydrogen bonds between water molecules are greater than the attractions between the water molecules and the walls of the container.
C) the viscosity of the water is greater than the viscosity of the glass.
D) surface tension of the water causes it to "bead up" inside the container.
E) the molecules are forced closer together because of London forces.
A) the attractive forces between the water molecules and the walls of the container are greater than the attractive forces between the water molecules.
B) the hydrogen bonds between water molecules are greater than the attractions between the water molecules and the walls of the container.
C) the viscosity of the water is greater than the viscosity of the glass.
D) surface tension of the water causes it to "bead up" inside the container.
E) the molecules are forced closer together because of London forces.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
8
Which group includes only endothermic processes?
A) freezing, vaporization, deposition
B) freezing, condensation, deposition
C) melting, condensation, deposition
D) melting, evaporation, sublimation
E) melting, condensation, sublimation
A) freezing, vaporization, deposition
B) freezing, condensation, deposition
C) melting, condensation, deposition
D) melting, evaporation, sublimation
E) melting, condensation, sublimation

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
9
Which description of the Clausius-Clapeyron equation is false?
A) graphing the equation produces a slope equal to -DHvap/R
B) based on the nonlinear increase in vapor pressure with increasing temperature
C) used only for calculating the enthalpy of vaporization of a substance
D) predicts a linear relationship between lnP and 1/T
E) can help determine the vapor pressure of a substance at different temperatures.
A) graphing the equation produces a slope equal to -DHvap/R
B) based on the nonlinear increase in vapor pressure with increasing temperature
C) used only for calculating the enthalpy of vaporization of a substance
D) predicts a linear relationship between lnP and 1/T
E) can help determine the vapor pressure of a substance at different temperatures.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
10
Which ending statement is false? The vapor pressure
A) is higher when the evaporation rate of a liquid is higher.
B) decreases when liquid molecules have weaker intermolecular forces.
C) is an equilibrium partial pressure due to gas molecules.
D) increases when liquid molecules have more kinetic energy to escape the liquid phase.
E) at a given temperature is greater for a more volatile liquid than a less volatile liquid.
A) is higher when the evaporation rate of a liquid is higher.
B) decreases when liquid molecules have weaker intermolecular forces.
C) is an equilibrium partial pressure due to gas molecules.
D) increases when liquid molecules have more kinetic energy to escape the liquid phase.
E) at a given temperature is greater for a more volatile liquid than a less volatile liquid.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
11
Which statement about heat of vaporization and heat of condensation is correct?
A) The heat of condensation is equal in magnitude but opposite in sign when compared to the heat of vaporization.
B) The heat of vaporization represents the heat released when a gas becomes a liquid, and the heat of condensation represents the heat required for a liquid to become a gas.
C) There is no general relationship between these two quantities and the values depend on the substance involved.
D) The heat of vaporization is always less than the heat of condensation.
E) The heat of vaporization is exactly equal to the heat of condensation.
A) The heat of condensation is equal in magnitude but opposite in sign when compared to the heat of vaporization.
B) The heat of vaporization represents the heat released when a gas becomes a liquid, and the heat of condensation represents the heat required for a liquid to become a gas.
C) There is no general relationship between these two quantities and the values depend on the substance involved.
D) The heat of vaporization is always less than the heat of condensation.
E) The heat of vaporization is exactly equal to the heat of condensation.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
12
Viscosity of a liquid ____ as temperature decreases because ____.
A) cannot be predicted; viscosity is determined by more than just temperature
B) increases; the molecules have more kinetic energy at the new temperature
C) increases; the molecules have less kinetic energy at the new temperature
D) decreases; the molecules have more kinetic energy at the new temperature
E) decreases; the molecules have less kinetic energy at the new temperature
A) cannot be predicted; viscosity is determined by more than just temperature
B) increases; the molecules have more kinetic energy at the new temperature
C) increases; the molecules have less kinetic energy at the new temperature
D) decreases; the molecules have more kinetic energy at the new temperature
E) decreases; the molecules have less kinetic energy at the new temperature

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
13
Which property of a liquid is paired with an incorrect explanation for that property?
A) fluid, because the molecules retain some mobility
B) transmit pressure equally in all directions, because their molecules can move in all directions
C) capillary action, because the molecules are less attracted to each other than to the walls of the container
D) incompressible, because the molecules are relatively far apart
E) viscous, because the molecules can become entangled as they move past each other
A) fluid, because the molecules retain some mobility
B) transmit pressure equally in all directions, because their molecules can move in all directions
C) capillary action, because the molecules are less attracted to each other than to the walls of the container
D) incompressible, because the molecules are relatively far apart
E) viscous, because the molecules can become entangled as they move past each other

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
14
When water is measured in a plastic graduated cylinder, a reverse meniscus is observed because
A) the hydrogen bonds between water molecules are greater than the attractions between the water molecules and the walls of the container.
B) the viscosity of the water is greater than the viscosity of the plastic.
C) surface tension of the water prevents it from "beading up" inside the container.
D) the molecules of water are forced closer together because of London forces.
E) the attractive forces between the water molecules and the walls of the container are greater than the attractive forces between the water molecules.
A) the hydrogen bonds between water molecules are greater than the attractions between the water molecules and the walls of the container.
B) the viscosity of the water is greater than the viscosity of the plastic.
C) surface tension of the water prevents it from "beading up" inside the container.
D) the molecules of water are forced closer together because of London forces.
E) the attractive forces between the water molecules and the walls of the container are greater than the attractive forces between the water molecules.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
15
A positive slope for a region of a heating curve indicates that ____ in that region.
A) no energy is being absorbed by the system
B) energy is being given off by the system, but it cannot be measured
C) energy is being absorbed by the system and is being used for a phase change
D) additional data is needed to explain this observation
E) energy is being absorbed by the system and is being used to increase the temperature
A) no energy is being absorbed by the system
B) energy is being given off by the system, but it cannot be measured
C) energy is being absorbed by the system and is being used for a phase change
D) additional data is needed to explain this observation
E) energy is being absorbed by the system and is being used to increase the temperature

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
16
Exhibit 11-1 For the following question(s), consider the graphic below: 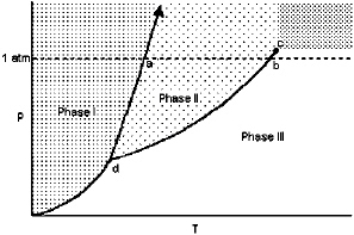 Refer to Exhibit 11-1. The temperature at point b is the
Refer to Exhibit 11-1. The temperature at point b is the
A) critical point.
B) triple point.
C) absolute freezing point.
D) normal freezing point.
E) normal boiling point.
 Refer to Exhibit 11-1. The temperature at point b is the
Refer to Exhibit 11-1. The temperature at point b is theA) critical point.
B) triple point.
C) absolute freezing point.
D) normal freezing point.
E) normal boiling point.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
17
Which group includes only exothermic processes?
A) freezing, vaporization, deposition
B) freezing, condensation, deposition
C) melting, condensation, deposition
D) melting, evaporation, sublimation
E) melting, condensation, sublimation
A) freezing, vaporization, deposition
B) freezing, condensation, deposition
C) melting, condensation, deposition
D) melting, evaporation, sublimation
E) melting, condensation, sublimation

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
18
The volatility of a liquid is increased by
A) decreasing the temperature and increasing the atmospheric pressure.
B) decreasing the temperature and decreasing the atmospheric pressure.
C) increasing the temperature and increasing the atmospheric pressure.
D) increasing the temperature and decreasing the atmospheric pressure.
E) decreasing the temperature and maintaining the atmospheric pressure.
A) decreasing the temperature and increasing the atmospheric pressure.
B) decreasing the temperature and decreasing the atmospheric pressure.
C) increasing the temperature and increasing the atmospheric pressure.
D) increasing the temperature and decreasing the atmospheric pressure.
E) decreasing the temperature and maintaining the atmospheric pressure.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
19
Which statement about heat of fusion and heat of condensation is correct?
A) There is no general relationship between these two quantities and the values depend on the substance involved.
B) The heat of fusion is always less than the heat of condensation.
C) The heat of fusion is exactly equal to the heat of condensation.
D) The heat of fusion represents the heat released when a gas becomes a liquid, and the heat of condensation represents the heat required for a liquid to become a gas.
E) The heat of condensation is equal in magnitude but opposite in sign when compared to the heat of fusion.
A) There is no general relationship between these two quantities and the values depend on the substance involved.
B) The heat of fusion is always less than the heat of condensation.
C) The heat of fusion is exactly equal to the heat of condensation.
D) The heat of fusion represents the heat released when a gas becomes a liquid, and the heat of condensation represents the heat required for a liquid to become a gas.
E) The heat of condensation is equal in magnitude but opposite in sign when compared to the heat of fusion.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
20
The normal boiling point of a liquid on a vapor pressure curve is found
A) at the point where the temperature is equal to the pressure.
B) at any point where the pressure is less than 760 mm Hg.
C) at the point where the temperature line crosses the 760 mm Hg line.
D) in the region below the curve.
E) in the region above the curve.
A) at the point where the temperature is equal to the pressure.
B) at any point where the pressure is less than 760 mm Hg.
C) at the point where the temperature line crosses the 760 mm Hg line.
D) in the region below the curve.
E) in the region above the curve.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
21
Which choice is an example of a metallic solid?
A) NaCl
B) crystal
C) iron
D) quartz
E) glass
A) NaCl
B) crystal
C) iron
D) quartz
E) glass

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
22
Exhibit 11-1 For the following question(s), consider the graphic below: 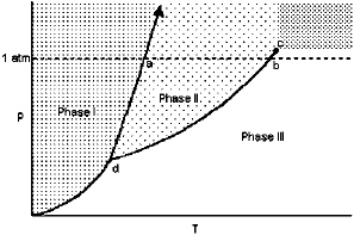 Refer to Exhibit 11-1. At a constant pressure of 1 atmosphere, the transition from Phase III to Phase II is
Refer to Exhibit 11-1. At a constant pressure of 1 atmosphere, the transition from Phase III to Phase II is
A) an exothermic process in which solid molecules lose energy
B) an exothermic process in which gas molecules lose energy
C) an endothermic process in which solid molecules gain energy
D) an endothermic process in which liquid molecules gain energy
E) an endothermic process in which gas molecules gain energy
 Refer to Exhibit 11-1. At a constant pressure of 1 atmosphere, the transition from Phase III to Phase II is
Refer to Exhibit 11-1. At a constant pressure of 1 atmosphere, the transition from Phase III to Phase II isA) an exothermic process in which solid molecules lose energy
B) an exothermic process in which gas molecules lose energy
C) an endothermic process in which solid molecules gain energy
D) an endothermic process in which liquid molecules gain energy
E) an endothermic process in which gas molecules gain energy

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
23
Which statement about network solids is false?
A) Graphite and diamond are examples of network solids.
B) Network solids consist of nonmetal atoms connected by covalent bonds.
C) Graphite is an example of a three-dimensional network solid.
D) All atoms in a network solid are connected to all other atoms through a network of bonds.
E) Silicates are an important class of network solids.
A) Graphite and diamond are examples of network solids.
B) Network solids consist of nonmetal atoms connected by covalent bonds.
C) Graphite is an example of a three-dimensional network solid.
D) All atoms in a network solid are connected to all other atoms through a network of bonds.
E) Silicates are an important class of network solids.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
24
Which choice is an example of a network solid?
A) NaCl
B) crystal
C) iron
D) quartz
E) glass
A) NaCl
B) crystal
C) iron
D) quartz
E) glass

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
25
Which description of the properties of water is false?
A) has hydrogen bonds and large enthalpy of vaporization
B) has covalent bonds and large enthalpy of fusion
C) has high specific heat capacity and low surface tension
D) has lower density as a solid than as a liquid and has large thermal conductivity
E) none of these are false
A) has hydrogen bonds and large enthalpy of vaporization
B) has covalent bonds and large enthalpy of fusion
C) has high specific heat capacity and low surface tension
D) has lower density as a solid than as a liquid and has large thermal conductivity
E) none of these are false

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
26
In face-centered cubic unit cells, ____ of the atom on each face of the unit cell is counted as part of that unit cell.
A) all
B) none
C) 1/2
D) 1/4
E) 1/8
A) all
B) none
C) 1/2
D) 1/4
E) 1/8

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
27
The density of water has its maximum value at 4 C because
A) the thermal motion of the molecules overcomes the hydrogen bonds and allows the molecules to approach each other more closely.
B) the thermal motion of the molecules is much greater than the energy of the hydrogen bonds and allows the molecules a great deal of freedom of motion.
C) covalent bonds begin breaking at this temperature.
D) the molecules are trapped in place by very strong hydrogen bonds.
E) the maximum number of hydrogen bonds exists between the molecules at this temperature.
A) the thermal motion of the molecules overcomes the hydrogen bonds and allows the molecules to approach each other more closely.
B) the thermal motion of the molecules is much greater than the energy of the hydrogen bonds and allows the molecules a great deal of freedom of motion.
C) covalent bonds begin breaking at this temperature.
D) the molecules are trapped in place by very strong hydrogen bonds.
E) the maximum number of hydrogen bonds exists between the molecules at this temperature.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
28
Exhibit 11-1 For the following question(s), consider the graphic below: 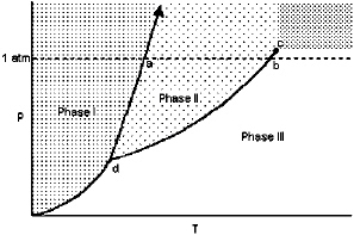 Refer to Exhibit 11-1. The temperature at point c is the
Refer to Exhibit 11-1. The temperature at point c is the
A) critical point.
B) triple point.
C) absolute freezing point.
D) normal freezing point.
E) normal boiling point.
 Refer to Exhibit 11-1. The temperature at point c is the
Refer to Exhibit 11-1. The temperature at point c is theA) critical point.
B) triple point.
C) absolute freezing point.
D) normal freezing point.
E) normal boiling point.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
29
Exhibit 11-1 For the following question(s), consider the graphic below: 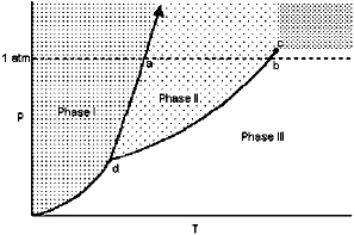 Refer to Exhibit 11-1. The transition from Phase I to Phase III is called
Refer to Exhibit 11-1. The transition from Phase I to Phase III is called
A) melting.
B) freezing.
C) sublimation.
D) evaporation.
E) condensation.
 Refer to Exhibit 11-1. The transition from Phase I to Phase III is called
Refer to Exhibit 11-1. The transition from Phase I to Phase III is calledA) melting.
B) freezing.
C) sublimation.
D) evaporation.
E) condensation.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
30
Which statement is false?
A) Amorphous solids have very little long-range order.
B) Ionic solids are poor conductors of electricity.
C) Metallic solids are good conductors of heat and electricity.
D) Molecular solids are held together by covalent bonds.
E) Network solids are bonded into infinite molecules of atoms by covalent bonds.
A) Amorphous solids have very little long-range order.
B) Ionic solids are poor conductors of electricity.
C) Metallic solids are good conductors of heat and electricity.
D) Molecular solids are held together by covalent bonds.
E) Network solids are bonded into infinite molecules of atoms by covalent bonds.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
31
A diatomic element is a shiny purple solid at room temperature that sublimes upon gentle heating. It is slightly soluble in water and considerably more soluble in alcohol. It does not conduct electricity as a solid, liquid or solution. This solid can best be classified as
A) ionic.
B) molecular.
C) amorphous.
D) metallic.
E) network.
A) ionic.
B) molecular.
C) amorphous.
D) metallic.
E) network.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
32
Which choice is an example of an ionic solid?
A) NaCl
B) crystal
C) iron
D) quartz
E) glass
A) NaCl
B) crystal
C) iron
D) quartz
E) glass

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
33
In an ionic compound such as NaCl, which consists of a fcc lattice of chloride ions, each sodium ion has ____ chloride ions surrounding it as nearest neighbors.
A) 8
B) 6
C) 4
D) 1
E) 0
A) 8
B) 6
C) 4
D) 1
E) 0

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
34
In body-centered cubic unit cells, ____ of the atom in the center of the unit cell is counted as part of that unit cell.
A) all
B) none
C) 1/2
D) 1/4
E) 1/8
A) all
B) none
C) 1/2
D) 1/4
E) 1/8

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
35
Which statement about crystalline solids is false?
A) All crystal lattices are three-dimensional repetitions of one of seven types of unit cells.
B) A unit cell is the smallest part of a crystalline lattice that can reproduce the entire crystal structure.
C) The three types of cubic unit cells are simple, body-centered, and face-centered.
D) For a given size atom or monatomic ion, a body-centered cubic lattice has a higher unit cell density than a face-centered cubic lattice.
E) A unit cell can contain nonmetal atoms, metal atoms, monatomic ions, polyatomic ions, or molecules.
A) All crystal lattices are three-dimensional repetitions of one of seven types of unit cells.
B) A unit cell is the smallest part of a crystalline lattice that can reproduce the entire crystal structure.
C) The three types of cubic unit cells are simple, body-centered, and face-centered.
D) For a given size atom or monatomic ion, a body-centered cubic lattice has a higher unit cell density than a face-centered cubic lattice.
E) A unit cell can contain nonmetal atoms, metal atoms, monatomic ions, polyatomic ions, or molecules.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
36
Which choice is best? The unusually high enthalpy of fusion, enthalpy of vaporization, and specific heat capacity of water impact our natural environment because these properties
A) prevent ice from sinking to the bottom of deep lakes in cold temperatures.
B) allow liquids to rise in plant stems through capillary action.
C) moderate temperature changes near large bodies of water.
D) cause water to be a greenhouse gas that contributes to natural global warming.
E) allow rapid transfer of thermal energy within living things.
A) prevent ice from sinking to the bottom of deep lakes in cold temperatures.
B) allow liquids to rise in plant stems through capillary action.
C) moderate temperature changes near large bodies of water.
D) cause water to be a greenhouse gas that contributes to natural global warming.
E) allow rapid transfer of thermal energy within living things.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
37
Exhibit 11-1 For the following question(s), consider the graphic below: 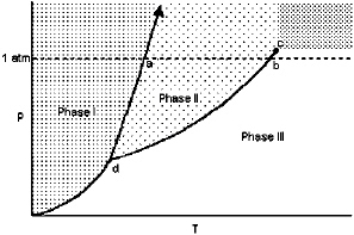 Refer to Exhibit 11-1. The transition from Phase I to Phase II is called
Refer to Exhibit 11-1. The transition from Phase I to Phase II is called
A) melting.
B) freezing.
C) sublimation.
D) evaporation.
E) condensation.
 Refer to Exhibit 11-1. The transition from Phase I to Phase II is called
Refer to Exhibit 11-1. The transition from Phase I to Phase II is calledA) melting.
B) freezing.
C) sublimation.
D) evaporation.
E) condensation.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
38
Which choice is an example of an amorphous solid?
A) NaCl
B) ionic solid
C) iron
D) quartz
E) glass
A) NaCl
B) ionic solid
C) iron
D) quartz
E) glass

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
39
The simplest explanation for the unusual properties of water in comparison to other compounds of similar structure and molar mass is
A) hydrogen bonding.
B) covalent bonding.
C) trigonal pyramidal structure.
D) London forces.
E) its purity.
A) hydrogen bonding.
B) covalent bonding.
C) trigonal pyramidal structure.
D) London forces.
E) its purity.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
40
Which choice is an example of a molecular solid?
A) NaCl
B) iodine
C) iron
D) quartz
E) glass
A) NaCl
B) iodine
C) iron
D) quartz
E) glass

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
41
According to band theory, a good conductor is a material which has a(n) ____ valence band, a(n) ____ conduction band, and ____ between them.
A) partially filled; partially filled; a wide gap
B) partially filled; empty; a narrow gap
C) partially filled; empty; no gap
D) completely filled; completely filled; a wide gap
E) empty; partially filled; a narrow gap
A) partially filled; partially filled; a wide gap
B) partially filled; empty; a narrow gap
C) partially filled; empty; no gap
D) completely filled; completely filled; a wide gap
E) empty; partially filled; a narrow gap

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
42
Which are properties of all metals (except Hg)?
I. Good conductors of heat and electricity
II. Easily formed into sheets or wires
III. Shiny
IV. Chemically unreactive
V. Soluble in water
A) all of these
B) none of these
C) I, II, III
D) I, II, IV
E) III, IV, V
I. Good conductors of heat and electricity
II. Easily formed into sheets or wires
III. Shiny
IV. Chemically unreactive
V. Soluble in water
A) all of these
B) none of these
C) I, II, III
D) I, II, IV
E) III, IV, V

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
43
According to band theory, a semiconductor is a material which has a(n) ____ valence band, a(n) ____ conduction band, and ____ between them.
A) completely filled; completely filled; a wide gap
B) empty; partially filled; a narrow gap
C) partially filled; partially filled; a wide gap
D) partially filled; empty; a narrow gap
E) partially filled; empty; no gap
A) completely filled; completely filled; a wide gap
B) empty; partially filled; a narrow gap
C) partially filled; partially filled; a wide gap
D) partially filled; empty; a narrow gap
E) partially filled; empty; no gap

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
44
According to band theory, an insulator is a material which has a(n) ____ valence band, a(n) ____ conduction band, and a ____ gap between them.
A) completely filled; completely filled; wide
B) empty; partially filled; narrow
C) partially filled; partially filled; wide
D) partially filled; empty; narrow
E) partially filled; empty; wide
A) completely filled; completely filled; wide
B) empty; partially filled; narrow
C) partially filled; partially filled; wide
D) partially filled; empty; narrow
E) partially filled; empty; wide

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
45
Which statement is false?
A) A material is conductive if enough electrons can move from the valence band into the conduction band.
B) Semiconductors become conductors at higher temperatures or in an electric field.
C) Electrons in a metal flow toward the positive end of an electrical field.
D) An energy band is a group of orbitals whose energy levels are widely spaced.
E) The energy level of the conduction band always overlaps the energy level of the valence band.
A) A material is conductive if enough electrons can move from the valence band into the conduction band.
B) Semiconductors become conductors at higher temperatures or in an electric field.
C) Electrons in a metal flow toward the positive end of an electrical field.
D) An energy band is a group of orbitals whose energy levels are widely spaced.
E) The energy level of the conduction band always overlaps the energy level of the valence band.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
46
Which statement about metallic bonding is not correct?
A) In the presence of an electrical field, the electrons migrate toward the positive charge.
B) The valence electrons move freely around the nuclei, allowing conduction of heat.
C) The flexibility of metals results from the mobility of the electron charge, which allows the nuclei to be moved without disrupting the overall structure of the metal.
D) It describes the freely moving valence electrons as an "electron sea."
E) The metal nuclei behave as negatively charged ions.
A) In the presence of an electrical field, the electrons migrate toward the positive charge.
B) The valence electrons move freely around the nuclei, allowing conduction of heat.
C) The flexibility of metals results from the mobility of the electron charge, which allows the nuclei to be moved without disrupting the overall structure of the metal.
D) It describes the freely moving valence electrons as an "electron sea."
E) The metal nuclei behave as negatively charged ions.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck


