Deck 24: Polymer Materials: Synthetic and Biological
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/56
Play
Full screen (f)
Deck 24: Polymer Materials: Synthetic and Biological
1
In an electrically conducting polymer,what is a "hole"?
A)It is a region of negative charge.
B)It is a delocalized π electron.
C)It is an antibonding π molecular orbital.
D)It is a π molecular orbital from which a π electron has been abstracted.
E)It is an ion,such as I3-,added to increase the conductivity of the polymer.
A)It is a region of negative charge.
B)It is a delocalized π electron.
C)It is an antibonding π molecular orbital.
D)It is a π molecular orbital from which a π electron has been abstracted.
E)It is an ion,such as I3-,added to increase the conductivity of the polymer.
It is a π molecular orbital from which a π electron has been abstracted.
2
The polymer 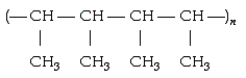 is formed by addition of which of the following?
is formed by addition of which of the following?
A)CH3CH2CH3
B)CH2 = CH - CH3
C)CH3CH = C(CH3)2
D)H2C = CH - CH - CH2
E)CH3 - CH = CH - CH3
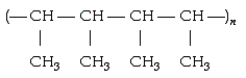 is formed by addition of which of the following?
is formed by addition of which of the following?A)CH3CH2CH3
B)CH2 = CH - CH3
C)CH3CH = C(CH3)2
D)H2C = CH - CH - CH2
E)CH3 - CH = CH - CH3
CH3 - CH = CH - CH3
3
The polymer formed from ethylene glycol (HOCH2CH2OH)and1,3-propanedicarboxylic acid (HOOCCH2COOH)is what type of polymer?
I.addition polymer
II.condensation polymer
III.homopolymer
IV.copolymer
A)II and IV
B)II and III
C)I and IV
D)I and III
E)None of these; it's a polyamide.
I.addition polymer
II.condensation polymer
III.homopolymer
IV.copolymer
A)II and IV
B)II and III
C)I and IV
D)I and III
E)None of these; it's a polyamide.
II and IV
4
What is the polymer formed by the condensation of ethylene glycol,HOCH2CH2OH,with 1,3-propanedicarboxylic acid,HOOCCH2COOH?
A)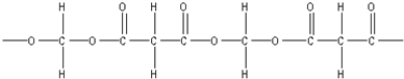
B)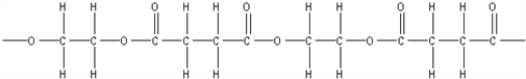
C)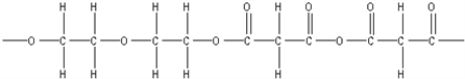
D)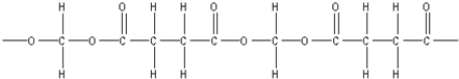
E)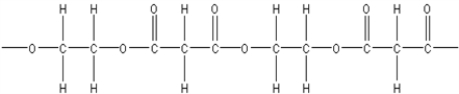
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following pairs of substances could form a polyester?
A)H2NCH2COOH + H2NCH2CH2COOH
B)HO(CH2)4COOH + HOCH2CH=CHCH3
C)HOCH2CH2OH + HOOCCOOH
D)H2C=CHCH3 + HOCH2CH2COOH
E)H2C=CHCN + H2C=CHCH3
A)H2NCH2COOH + H2NCH2CH2COOH
B)HO(CH2)4COOH + HOCH2CH=CHCH3
C)HOCH2CH2OH + HOOCCOOH
D)H2C=CHCH3 + HOCH2CH2COOH
E)H2C=CHCN + H2C=CHCH3

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
6
What of the following polymers could be produced by the free-radical addition of 1,1-difluoroethylene,F2C=CH2?
A)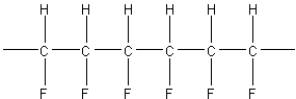
B)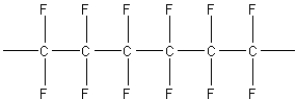
C)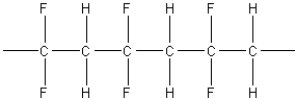
D)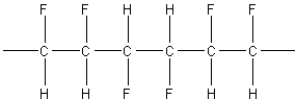
E)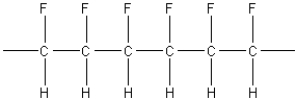
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is not an electrically conducting polymer?
A)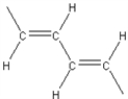
B)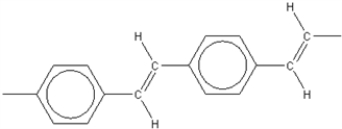
C)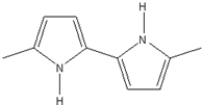
D)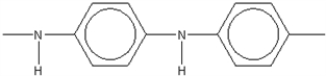
E)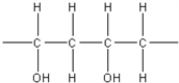
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements does not describe natural rubber?
A)It is a copolymer.
B)Isoprene is the monomer.
C)It may be sticky unless it has been vulcanized.
D)It is a homopolymer.
E)It can be made from the sap of a certain tree.
A)It is a copolymer.
B)Isoprene is the monomer.
C)It may be sticky unless it has been vulcanized.
D)It is a homopolymer.
E)It can be made from the sap of a certain tree.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
9
For a free-radical addition polymerization,which of the following statements is true?
A)Upon polymerization,σ-bonds are transformed into π-bonds.
B)The final polymer is a free radical.
C)A copolymer is formed between the monomer and the initiator.
D)Upon polymerization,π-bonds are transformed into σ-bonds.
E)The final polymer must be electrically conducting.
A)Upon polymerization,σ-bonds are transformed into π-bonds.
B)The final polymer is a free radical.
C)A copolymer is formed between the monomer and the initiator.
D)Upon polymerization,π-bonds are transformed into σ-bonds.
E)The final polymer must be electrically conducting.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
10
What is the type of polymer formed when 1,5-pentanediol,HOCH2CH2CH2CH2CH2OH,reacts with 1,6-hexanedicarboxylic acid,HOOCCH2CH2CH2CH2COOH?
A)polyacetylene
B)polyester
C)polyamide
D)polypropylene
E)polyolefin
A)polyacetylene
B)polyester
C)polyamide
D)polypropylene
E)polyolefin

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
11
The amino acid L-lysine has the amine side chain R = −(CH2)4NH2.At low pH,all ionizable groups are protonated.What is the net charge on this fully protonated form of L-lysine?
A)2
B)1
C)−1
D)−2
E)0
A)2
B)1
C)−1
D)−2
E)0

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following combinations can produce a condensation polymer? (R and R' are alkyl groups)
A)R−OH + HOOC−R'−COOH
B)HO−R−OH + R'−COOH
C)H2N−R−NH2 + HOOC−R'−COOH
D)A and C
E)A,B,and C
A)R−OH + HOOC−R'−COOH
B)HO−R−OH + R'−COOH
C)H2N−R−NH2 + HOOC−R'−COOH
D)A and C
E)A,B,and C

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
13
What is the type of polymer formed when 1,4-butanecarboxylic acid,HOOCCH2CH2COOH,reacts with 1,4-diaminobutane,H2NCH2CH2CH2CH2NH2?
A)polyacetylene
B)polyolefin
C)polystyrene
D)polyester
E)polyamide
A)polyacetylene
B)polyolefin
C)polystyrene
D)polyester
E)polyamide

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
14
What is the polymer formed by the condensation of 1,3-propanedicarboxylic acid,HOOCCH2COOH,with 1,2-diaminoethane,NH2CH2CH2NH2?
A)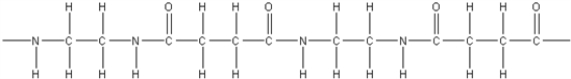
B)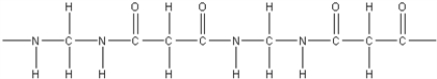
C)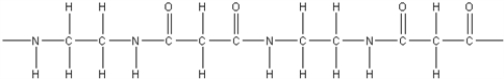
D)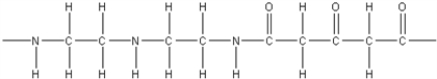
E)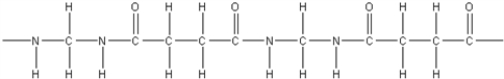
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
15
Consider the polymer drawn below: 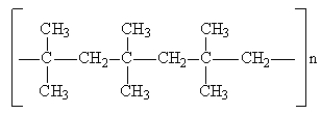 What monomer(s)could produce the above polymer?
What monomer(s)could produce the above polymer?
A)CH2 = CH2 and CH3CH = CH2
B)CH3CH = CHCH3
C)CH2 = C(CH3)2
D)CO and CH2 = CH2
E)none of these
 What monomer(s)could produce the above polymer?
What monomer(s)could produce the above polymer?A)CH2 = CH2 and CH3CH = CH2
B)CH3CH = CHCH3
C)CH2 = C(CH3)2
D)CO and CH2 = CH2
E)none of these

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is not an addition polymer?
A)poly(vinyl chloride)
B)polyethylene
C)polystyrene
D)polypropylene
E)polyester
A)poly(vinyl chloride)
B)polyethylene
C)polystyrene
D)polypropylene
E)polyester

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
17
The side chain on the amino acid phenylalanine 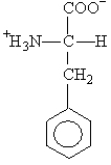 is classified as
is classified as
A)polar.
B)asymmetric.
C)hydrophilic.
D)nonpolar.
E)chiral.
 is classified as
is classified asA)polar.
B)asymmetric.
C)hydrophilic.
D)nonpolar.
E)chiral.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
18
For the addition polymer polyacrylonitrile shown below,what is the chemical structure of the monomer? 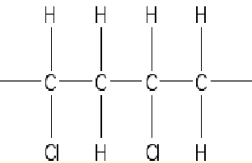
A)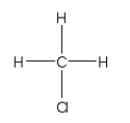
B)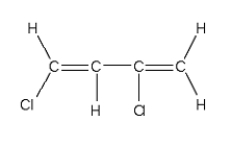
C)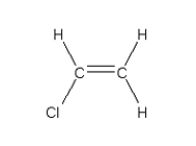
D)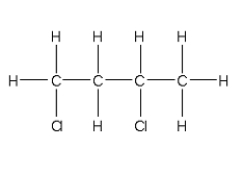
E)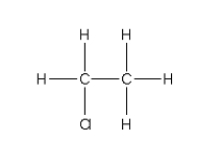

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
19
Elimination of a small molecule such as water is involved in formation of which type of polymer?
A)condensation polymer
B)addition polymer
C)free-radical polymer
D)homopolymer
E)copolymer
A)condensation polymer
B)addition polymer
C)free-radical polymer
D)homopolymer
E)copolymer

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
20
The structure of the polymer used in a freezer wrap can mainly be described as follows:
[CCl2 -CH2 -CCl2 -CH2 -CCl2 -CH2 -CCl2 -CH2]n
Which monomer could produce this polymer?
A)Cl2C - CH2
B)CCl2
C)CCl2 = CH2
D)Cl2C = CH2 = CCl2
E)none of these
[CCl2 -CH2 -CCl2 -CH2 -CCl2 -CH2 -CCl2 -CH2]n
Which monomer could produce this polymer?
A)Cl2C - CH2
B)CCl2
C)CCl2 = CH2
D)Cl2C = CH2 = CCl2
E)none of these

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
21
The side chain on the amino acid tyrosine 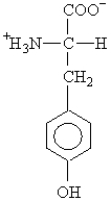 is classified as
is classified as
A)hydrophobic.
B)nonpolar.
C)a zwitterion.
D)polar.
E)asymmetric.
 is classified as
is classified asA)hydrophobic.
B)nonpolar.
C)a zwitterion.
D)polar.
E)asymmetric.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
22
A fragment of a DNA molecule has the base sequence TGAC.What is the complementary sequence?
A)GTCA
B)CAGT
C)AGTC
D)ACTG
E)TGAC
A)GTCA
B)CAGT
C)AGTC
D)ACTG
E)TGAC

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is the zwitterionic form of glycine (R = H)?
A)(-NHCH2CO2H)
B)NH2CHCO2H+
C)(+NH2CH2CO2-)
D)(+NH3CH2CO2-)
E)(NH2CH2CO2-)
A)(-NHCH2CO2H)
B)NH2CHCO2H+
C)(+NH2CH2CO2-)
D)(+NH3CH2CO2-)
E)(NH2CH2CO2-)

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following represents the linkage connecting amino acids in proteins?
A)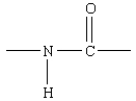
B)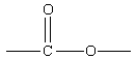
C)
D)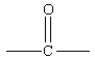
E)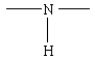
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is an amino acid?
A)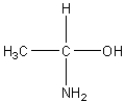
B)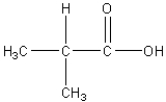
C)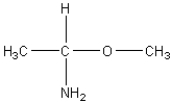
D)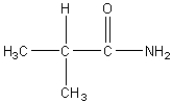
E)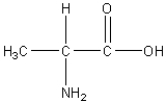
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
26
How many different tripeptides can be made from two molecules of the amino acid valine (val)and one molecule of the amino acid alanine (ala)?
A)4
B)3
C)1
D)5 or more
E)2
A)4
B)3
C)1
D)5 or more
E)2

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
27
The nucleic acid sequence that is complementary to the DNA sequence GAC TAC GTT AGC is
A)CTG ATG CAA TCG.
B)GAC TAC GTT AGC.
C)CGA TTG CAT CAG.
D)TCA GCA TGG CTA.
E)none of these
A)CTG ATG CAA TCG.
B)GAC TAC GTT AGC.
C)CGA TTG CAT CAG.
D)TCA GCA TGG CTA.
E)none of these

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following amino acids has a hydrophilic side chain?
A)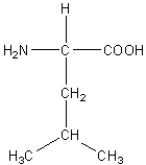
B)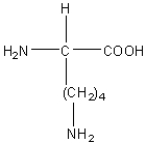
C)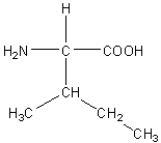
D)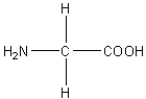
E)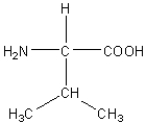
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
29
Polymers of amino-acid units are called
A)metabolites.
B)nucleic acids.
C)lipids.
D)carbohydrates.
E)proteins.
A)metabolites.
B)nucleic acids.
C)lipids.
D)carbohydrates.
E)proteins.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
30
What is the functional group corresponding to a peptide bond?
A)amine
B)ether
C)amide
D)hemiacetal
E)carboxylic acid
A)amine
B)ether
C)amide
D)hemiacetal
E)carboxylic acid

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
31
When L-lysine (R = −(CH2)4NH2)is reacted with a large excess of strong base,which of the following ions is the predominant species in solution?
A)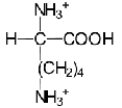
B)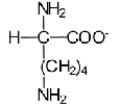
C)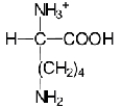
D)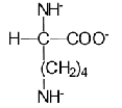
E)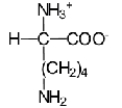
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
32
Near a pH of 5.5,L-lysine (R = −(CH2)4NH2)the major species in aqueous solution is the diprotonated zwitterion.Given the following acid dissociation constants,what is the correct structure of the zwitterion?
Functional Group
Ka
Carboxylic acid
1)7 x10-2
Amine
8)5 x10-10
Side chain amine
1)51 x10-11
A)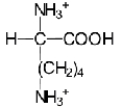
B)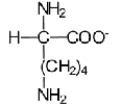
C)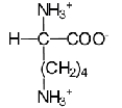
D)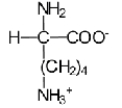
E)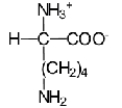
Functional Group
Ka
Carboxylic acid
1)7 x10-2
Amine
8)5 x10-10
Side chain amine
1)51 x10-11
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
33
The sequence of amino acids held together by peptide bonds in a protein is the
A)tertiary structure.
B)hydrogen bonding.
C)primary structure.
D)quaternary structure.
E)secondary structure.
A)tertiary structure.
B)hydrogen bonding.
C)primary structure.
D)quaternary structure.
E)secondary structure.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following amino acids has a hydrophobic side chain?
A)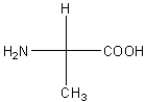
B)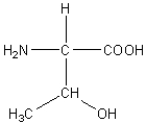
C)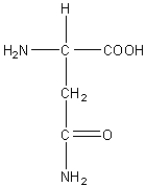
D)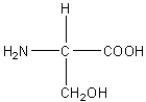
E)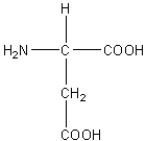
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following structures is the best representation for the amino acid alanine under physiological conditions?
A)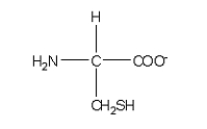
B)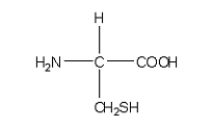
C)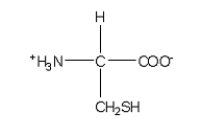
D)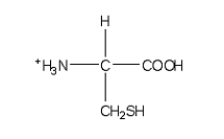
E)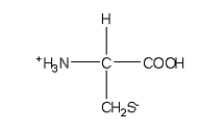
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
36
What are the building blocks of nucleic acids?
A)proteins
B)monosaccharides
C)lipids
D)nucleotides
E)amino acids
A)proteins
B)monosaccharides
C)lipids
D)nucleotides
E)amino acids

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
37
The overall shape of a protein is maintained by
A)hydrogen bonding.
B)ionic bonds.
C)dipole-dipole bonding.
D)covalent bonds.
E)all of these
A)hydrogen bonding.
B)ionic bonds.
C)dipole-dipole bonding.
D)covalent bonds.
E)all of these

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
38
The alpha helix and beta sheet are examples of
A)protein denaturation.
B)protein primary structure.
C)complementary bases.
D)protein tertiary structure.
E)protein secondary structure.
A)protein denaturation.
B)protein primary structure.
C)complementary bases.
D)protein tertiary structure.
E)protein secondary structure.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
39
A hemiacetal is formed from
A)an alcohol and a ketone.
B)a carboxylic acid and a mercaptan.
C)an alcohol and an ether.
D)an aldehyde and an alcohol.
E)an ester and an amine.
A)an alcohol and a ketone.
B)a carboxylic acid and a mercaptan.
C)an alcohol and an ether.
D)an aldehyde and an alcohol.
E)an ester and an amine.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following statements is correct?
A)Fibrous proteins tend to form long,water-soluble fibers,while globular proteins tend to form compact,water-soluble shapes.
B)Fibrous proteins tend to form long,water-insoluble fibers,while globular proteins tend to form compact,water-soluble shapes.
C)Fibrous proteins tend to form long,water-insoluble fibers,while globular proteins tend to form compact,water-insoluble shapes.
D)Both fibrous proteins and globular proteins tend to form water-soluble structures of about the same shape.
E)Fibrous proteins tend to form long,water-soluble fibers,while globular proteins tend to form compact,water-insoluble shapes.
A)Fibrous proteins tend to form long,water-soluble fibers,while globular proteins tend to form compact,water-soluble shapes.
B)Fibrous proteins tend to form long,water-insoluble fibers,while globular proteins tend to form compact,water-soluble shapes.
C)Fibrous proteins tend to form long,water-insoluble fibers,while globular proteins tend to form compact,water-insoluble shapes.
D)Both fibrous proteins and globular proteins tend to form water-soluble structures of about the same shape.
E)Fibrous proteins tend to form long,water-soluble fibers,while globular proteins tend to form compact,water-insoluble shapes.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
41
What is the cell structure that contains the cell's DNA?
A)chromosome
B)codon
C)ribosome
D)gene
E)nucleus
A)chromosome
B)codon
C)ribosome
D)gene
E)nucleus

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following units is not found in a DNA molecule?
A)a phosphate group
B)a peptide bond
C)an organic base
D)a five-membered ring
E)a ribose sugar
A)a phosphate group
B)a peptide bond
C)an organic base
D)a five-membered ring
E)a ribose sugar

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
43
A compound used to make a polymer and from which the polymer's unit arises is called a _____.
A)amine
B)monomer
C)amide
D)hydroxide
E)alkane
A)amine
B)monomer
C)amide
D)hydroxide
E)alkane

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
44
Double stranded DNA may be represented as a double helix with the two strands held together by
A)hydrogen bonds.
B)covalent bonds.
C)disulfide bonds.
D)hydrophobic bonds.
E)ionic bonds.
A)hydrogen bonds.
B)covalent bonds.
C)disulfide bonds.
D)hydrophobic bonds.
E)ionic bonds.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is(are)not necessary for protein synthesis at the time and place where synthesis occurs?
A)ribosomes
B)DNA
C)mRNA
D)amino acids
E)tRNA
A)ribosomes
B)DNA
C)mRNA
D)amino acids
E)tRNA

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
46
Which type of molecule attaches itself to a ribosome,where it serves as a pattern for protein biosynthesis?
A)transfer RNA
B)protein
C)DNA
D)ribosomal RNA
E)messenger RNA
A)transfer RNA
B)protein
C)DNA
D)ribosomal RNA
E)messenger RNA

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
47
Which type of molecule is responsible for carrying an amino acid to a ribosome?
A)protein
B)DNA
C)ribosomal RNA
D)messenger RNA
E)transfer RNA
A)protein
B)DNA
C)ribosomal RNA
D)messenger RNA
E)transfer RNA

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following 2'-deoxynucleotides contains the base thymine?
A)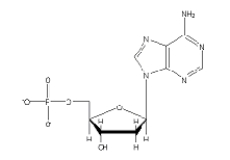
B)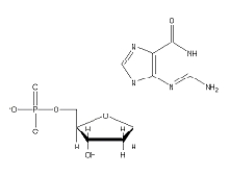
C)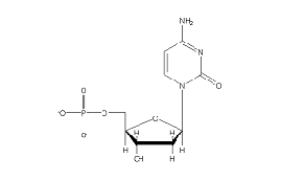
D)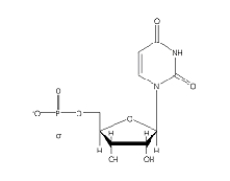
E)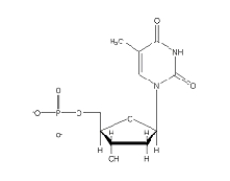
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
49
A hemiketal is formed from a(n)_____.
A)alcohol and an aldehyde
B)ketone and an ether
C)ketone and an alcohol
D)alcohol and an ester
E)ether and an ester
A)alcohol and an aldehyde
B)ketone and an ether
C)ketone and an alcohol
D)alcohol and an ester
E)ether and an ester

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
50
How many RNA nucleotides are in a codon?
A)1
B)5
C)2
D)4
E)3
A)1
B)5
C)2
D)4
E)3

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
51
What is the charge on each phosphate group in DNA in an aqueous solution near physiological pH?
A)0
B)1
C)2
D)−1
E)−2
A)0
B)1
C)2
D)−1
E)−2

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
52
A(n)_____ is a linear polymer of nucleotide units linked from the hydroxyl group at the 3' carbon of the pentose of one nucleotide to the phosphate group of the other nucleotide.
A)ribonucleotide
B)polysaccharide
C)polynucleotide
D)ketopentose
E)aldopentose
A)ribonucleotide
B)polysaccharide
C)polynucleotide
D)ketopentose
E)aldopentose

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
53
What is the nitrogenous base that is found in RNA but not in DNA?
A)thymine
B)guanine
C)uracil
D)cytosine
E)adenine
A)thymine
B)guanine
C)uracil
D)cytosine
E)adenine

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
54
Where in the cell does protein synthesis cell take place?
A)ribosome
B)nucleus
C)gene
D)chromosome
E)codon
A)ribosome
B)nucleus
C)gene
D)chromosome
E)codon

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
55
What is the significance of a "stop codon"?
A)When it appears in messenger RNA,it is a signal to stop protein biosynthesis.
B)When it appears in transfer RNA,it is a signal to stop transferring amino acids to the growing protein chain.
C)When it appears in DNA,it is a signal to stop chromosome biosynthesis.
D)When it appears in a protein,it is a signal for the protein to unfold.
E)When it appears in ribosomal RNA,it is a signal to stop catalyzing protein biosynthesis.
A)When it appears in messenger RNA,it is a signal to stop protein biosynthesis.
B)When it appears in transfer RNA,it is a signal to stop transferring amino acids to the growing protein chain.
C)When it appears in DNA,it is a signal to stop chromosome biosynthesis.
D)When it appears in a protein,it is a signal for the protein to unfold.
E)When it appears in ribosomal RNA,it is a signal to stop catalyzing protein biosynthesis.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
56
A(n)_____ is a chemical species of very high molecular weight made up from many units of low molecular weight covalently linked together.
A)polymer
B)amine
C)monomer
D)hydroxide
E)alkane
A)polymer
B)amine
C)monomer
D)hydroxide
E)alkane

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck


