Deck 5: The Gaseous State
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/104
Play
Full screen (f)
Deck 5: The Gaseous State
1
A particular gas exerts a pressure of 4.33 bar.What is this pressure in units of atmospheres?
A)4.38 atm
B)3.25 × 103 atm
C)3.33 × 103 atm
D)4.27 atm
E)4.33 atm
A)4.38 atm
B)3.25 × 103 atm
C)3.33 × 103 atm
D)4.27 atm
E)4.33 atm
4.27 atm
2
A flexible vessel contains 43 L of gas where the pressure is 1.3 atm.What will the volume be when the pressure is 0.61 atm,the temperature remaining constant?
A)0.011 L
B)20 L
C)43 L
D)0.044 L
E)92 L
A)0.011 L
B)20 L
C)43 L
D)0.044 L
E)92 L
92 L
3
A particular gas exerts a pressure of 2.52 bar.What is this pressure in units of pascals?
A)2.49 × 105 Pa
B)2.52 × 10-5 Pa
C)2.52 × 105 Pa
D)2.55 × 105 Pa
E)2.49 × 10-5 Pa
A)2.49 × 105 Pa
B)2.52 × 10-5 Pa
C)2.52 × 105 Pa
D)2.55 × 105 Pa
E)2.49 × 10-5 Pa
2.52 × 105 Pa
4
A 2.00-L glass soda bottle filled only with air is tightly capped at 25°C and 728.0 mmHg.If the bottle is placed in water at 65°C,what is the pressure in the bottle?
A)280 mmHg
B)826 mmHg
C)1890 mmHg
D)642 mmHg
E)324 mmHg
A)280 mmHg
B)826 mmHg
C)1890 mmHg
D)642 mmHg
E)324 mmHg

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
5
A flexible vessel contains 78.00 L of gas at a pressure of 1.50 atm.Under conditions of constant temperature and moles of gas,what is the pressure of the gas when the volume of the vessel is tripled?
A)0.572 atm
B)4.5111 atm
C)2.38 atm
D)1.5 atm
E)0.025578 atm
A)0.572 atm
B)4.5111 atm
C)2.38 atm
D)1.5 atm
E)0.025578 atm

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
6
Absolute zero is the point at which
A)a straight-line graph of V versus T (°C)intersects the origin.
B)a straight-line graph of 1/V versus P at constant T intersects the origin.
C)gaseous helium liquefies.
D)a straight-line graph of V versus 1/P at constant T intersects the origin.
E)a straight-line graph of V versus T (K)intersects the origin.
A)a straight-line graph of V versus T (°C)intersects the origin.
B)a straight-line graph of 1/V versus P at constant T intersects the origin.
C)gaseous helium liquefies.
D)a straight-line graph of V versus 1/P at constant T intersects the origin.
E)a straight-line graph of V versus T (K)intersects the origin.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
7
A particular gas exerts a pressure of 4.09 × 104 Pa.What is this pressure in units of atmospheres?
A)0.403 atm
B)4.04 × 109 atm
C)0.414 atm
D)0.409 atm
E)4.14 × 109 atm
A)0.403 atm
B)4.04 × 109 atm
C)0.414 atm
D)0.409 atm
E)4.14 × 109 atm

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
8
A flexible vessel is filled to a certain pressure with 28.00 L of gas.Under conditions of constant temperature and moles of gas,how does the pressure of the gas change when the volume of the gas is tripled?
A)The pressure decreases by a factor of three.
B)The pressure increases by a factor of two.
C)The pressure remains the same.
D)The pressure decreases by a factor of two.
E)The pressure decreases by a factor of four.
A)The pressure decreases by a factor of three.
B)The pressure increases by a factor of two.
C)The pressure remains the same.
D)The pressure decreases by a factor of two.
E)The pressure decreases by a factor of four.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
9
The pressure of a certain gas is measured to be 264.6 mmHg.What is this pressure expressed in units of pascals?
A)3.436 × 10-6 Pa
B)2.91 × 105 Pa
C)2.835 × 10-5 Pa
D)2.681 × 107 Pa
E)3.528 × 104 Pa
A)3.436 × 10-6 Pa
B)2.91 × 105 Pa
C)2.835 × 10-5 Pa
D)2.681 × 107 Pa
E)3.528 × 104 Pa

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
10
It is possible to make a barometer using a liquid other than mercury.What would be the height (in meters)of a column of dichloromethane at a pressure of 0.830 atm,given that 0.830 atm is equal to a 0.63522 m column of mercury and the densities of mercury and dichloromethane are 13.5 g/cm3 and 1.33 g/cm3,respectively.
A)6.4641 m
B)0.156 m
C)0.0621 m
D)0.838 m
E)1.19 m
A)6.4641 m
B)0.156 m
C)0.0621 m
D)0.838 m
E)1.19 m

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
11
A particular gas exerts a pressure of 689 mmHg.What is this pressure in units of bar?
A)5.17 × 105 bar
B)5.31 × 105 bar
C)698 bar
D)0.894 bar
E)0.918 bar
A)5.17 × 105 bar
B)5.31 × 105 bar
C)698 bar
D)0.894 bar
E)0.918 bar

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
12
A particular gas exerts a pressure of 4.02 atm.What is this pressure in units of bar?
A)3.06 × 103 bar
B)4.02 × 105 bar
C)4.07 bar
D)3.96 bar
E)5.29 × 10-3 bar
A)3.06 × 103 bar
B)4.02 × 105 bar
C)4.07 bar
D)3.96 bar
E)5.29 × 10-3 bar

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
13
A particular gas exerts a pressure of 731 mmHg.What is this pressure in units of atmospheres?
A)5.48 × 105 atm
B)5.63 × 105 atm
C)721 atm
D)0.974 atm
E)0.961 atm
A)5.48 × 105 atm
B)5.63 × 105 atm
C)721 atm
D)0.974 atm
E)0.961 atm

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
14
The following volume-temperature plots were made at different values of constant pressure while the number of moles of gas in each experiment remained the same.Which plot represents measurements at the highest pressure? 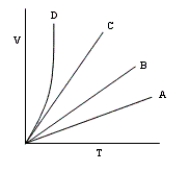
A)B
B)C
C)A
D)They are all at the same pressure.
E)D

A)B
B)C
C)A
D)They are all at the same pressure.
E)D

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
15
A sample of methane,CH4,occupies a volume of 318.0 mL at 25°C and exerts a pressure of 1010.0 mmHg.If the volume of the gas is allowed to expand to 770.0 mL at 298 K,what will be the pressure of the gas?
A)2440 mmHg
B)4970 mmHg
C)417.1 mmHg
D)0.01727 mmHg
E)452 mmHg
A)2440 mmHg
B)4970 mmHg
C)417.1 mmHg
D)0.01727 mmHg
E)452 mmHg

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
16
The pressure of a certain gas is measured to be 4.304 × 103 Pa.What is this pressure expressed in units of mmHg?
A)5.589 × 10-5 mmHg
B)3.271 × 106 mmHg
C)3.098 × 10-2 mmHg
D)32.28 mmHg
E)1.789 × 104 mmHg
A)5.589 × 10-5 mmHg
B)3.271 × 106 mmHg
C)3.098 × 10-2 mmHg
D)32.28 mmHg
E)1.789 × 104 mmHg

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
17
A particular gas exerts a pressure of 5.56 × 104 Pa.What is this pressure in units of bar?
A)5.56 × 109 bar
B)0.556 bar
C)73.1 bar
D)5.49 ×104 bar
E)5.63 × 104 bar
A)5.56 × 109 bar
B)0.556 bar
C)73.1 bar
D)5.49 ×104 bar
E)5.63 × 104 bar

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
18
A particular gas exerts a pressure of 2.42 atm.What is this pressure in units of mmHg?
A)2.38 mmHg
B)1.84 × 103 mmHg
C)2.45 mmHg
D)3.18 × 10-3 mmHg
E)1.86 × 103 mmHg
A)2.38 mmHg
B)1.84 × 103 mmHg
C)2.45 mmHg
D)3.18 × 10-3 mmHg
E)1.86 × 103 mmHg

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
19
When the valve between the 2.00-L bulb,in which the gas pressure is 2.50 atm,and the 3.00-L bulb,in which the gas pressure is 1.50 atm,is opened,what will be the final pressure in the two bulbs? Assume the temperature remains constant. 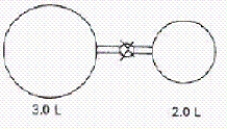
A)1.90 atm
B)4.00 atm
C)2.17 atm
D)2.10 atm
E)1.83 atm

A)1.90 atm
B)4.00 atm
C)2.17 atm
D)2.10 atm
E)1.83 atm

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
20
A flexible vessel contains 37 L of gas where the pressure is 1.0 atm.What will the volume be when the pressure is 0.70 atm,the temperature remaining constant?
A)0.019 L
B)26 L
C)37 L
D)0.046 L
E)53 L
A)0.019 L
B)26 L
C)37 L
D)0.046 L
E)53 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
21
A flexible container is charged with a certain volume of gas at 315.0 Κ.Under conditions of constant pressure and moles of gas,how does the temperature of the gas change when the volume is tripled?
A)The temperature increases by a factor of three.
B)The temperature decreases by a factor of four.
C)The temperature decreases by a factor of two.
D)The temperature increases by a factor of four.
E)The temperature increases by a factor of two.
A)The temperature increases by a factor of three.
B)The temperature decreases by a factor of four.
C)The temperature decreases by a factor of two.
D)The temperature increases by a factor of four.
E)The temperature increases by a factor of two.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
22
What volume of sulfur trioxide gas,SO3,has the same number of atoms as 4 L of helium gas at the same temperature and pressure?
A)20 L
B)0.8 L
C)4 L
D)1 L
E)16 L
A)20 L
B)0.8 L
C)4 L
D)1 L
E)16 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
23
What volume of gaseous oxygen,O2,has the same moles of gas as 3 L of argon gas at the same temperature and pressure?
A)9 L
B)1 L
C)3 L
D)1.5 L
E)6 L
A)9 L
B)1 L
C)3 L
D)1.5 L
E)6 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
24
A given mass of gas occupies a volume of 4.00 L at 60°C and 550 mmHg.Which of the following mathematical expressions will yield its temperature at 2.50 L and 900 mmHg?
A)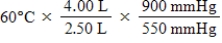
B)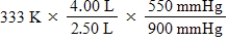
C)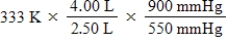
D)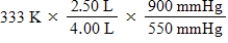
E)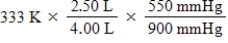
A)
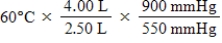
B)
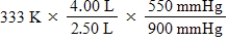
C)
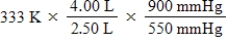
D)
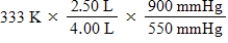
E)
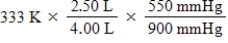

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
25
A gas occupies a volume of 2.00 L at 780 mmHg and 70.0°C.Which of the following mathematical expressions will yield its volume at STP?
A)
B)
C)
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
26
A gas occupies a volume of 2.75 L at 350 mmHg and 200°C.Which mathematical expression gives the correct volume at 600 mmHg and 250°C?
A)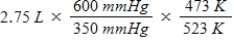
B)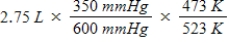
C)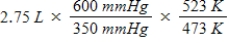
D)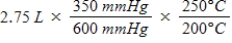
E)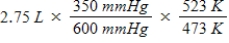
A)
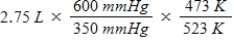
B)
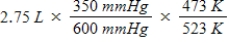
C)
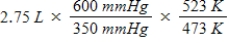
D)
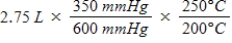
E)
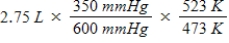

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is a correct statement of Charles's law, 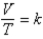 ?
?
A)The volume of a gas varies proportionally with the pressure.
B)The volume of a gas sample varies directly with the absolute temperature.
C)All gas samples of the same volume at STP contain the same number of atoms.
D)The pressure of a gas sample varies inversely with the volume.
E)All gas samples of the same volume at STP contain the same number of molecules.
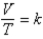 ?
?A)The volume of a gas varies proportionally with the pressure.
B)The volume of a gas sample varies directly with the absolute temperature.
C)All gas samples of the same volume at STP contain the same number of atoms.
D)The pressure of a gas sample varies inversely with the volume.
E)All gas samples of the same volume at STP contain the same number of molecules.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
28
A flexible container is charged with 79.00 L of gas at 352 Κ.Under conditions of constant pressure and moles of gas,what is the volume of the gas when the temperature is decreased by a factor of three?
A)26.3 L
B)237 L
C)0.00421 L
D)79 L
E)11.8 L
A)26.3 L
B)237 L
C)0.00421 L
D)79 L
E)11.8 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
29
A fixed amount of gas in a rigid container is heated from 300 to 600 K.Which of the following responses best describes what will happen to the pressure of the gas?
A)The pressure will increase by a factor of 2.
B)The pressure will increase by a factor less than 2.
C)The pressure will increase by a factor greater than 2.
D)The pressure will decrease by a factor of 2.
E)The pressure will remain the same.
A)The pressure will increase by a factor of 2.
B)The pressure will increase by a factor less than 2.
C)The pressure will increase by a factor greater than 2.
D)The pressure will decrease by a factor of 2.
E)The pressure will remain the same.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
30
A 1.00-L bulb contains a sample of O2 at 25°C and 1.00 atm pressure.A second 1.00-L bulb contains a sample of CH4 at 25°C and 1.00 atm pressure.What is the ratio of the number of molecules of methane to the number of molecules of oxygen in each of the containers?
A)2:5
B)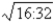
C)5:2
D)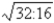
E)1:1
A)2:5
B)
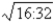
C)5:2
D)
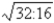
E)1:1

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
31
A fixed amount of gas in a rigid container is heated from 100°C to 600°C.Which of the following responses best describes what will happen to the pressure of the gas?
A)The pressure will increase by a factor greater than 6.
B)The pressure will increase by a factor of 6.
C)The pressure will increase by a factor less than 6.
D)The pressure will decrease by a factor of 6.
E)The pressure will remain the same.
A)The pressure will increase by a factor greater than 6.
B)The pressure will increase by a factor of 6.
C)The pressure will increase by a factor less than 6.
D)The pressure will decrease by a factor of 6.
E)The pressure will remain the same.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
32
The pressure of 6.2 L of nitrogen gas in a flexible container is decreased to one-half its original pressure,and its absolute temperature is increased to double the original temperature.The volume is now
A)3.1 L.
B)6.2 L.
C)12 L.
D)25 L.
E)1.6 L.
A)3.1 L.
B)6.2 L.
C)12 L.
D)25 L.
E)1.6 L.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
33
A rigid container is charged with a gas to a pressure of 740 mmHg at 40.0°C and tightly sealed.If the temperature of the gas increases by 80.0°C what is the new pressure?
A)1480 mmHg
B)589 mmHg
C)551 mmHg
D)929 mmHg
E)1110 mmHg
A)1480 mmHg
B)589 mmHg
C)551 mmHg
D)929 mmHg
E)1110 mmHg

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
34
A gas occupying a volume of 1.50 L exerts a pressure of 700 mmHg at 200°C.Which mathematical expression gives the correct pressure at 3.50 L and 350°C?
A)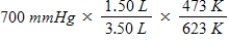
B)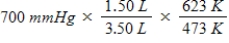
C)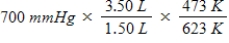
D)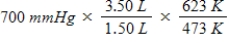
E)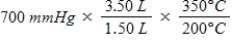
A)
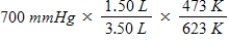
B)
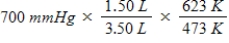
C)
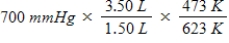
D)
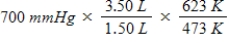
E)
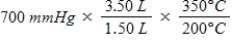

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following statements,concerning equal volumes of the gases dinitrogen monoxide,N2O,and propane,C3H8,at the same temperature and pressure,is not correct?
A)The moles of N2O and C3H8 are equal.
B)They have the same density.
C)They have the same number of molecules.
D)They have the same number of atoms.
E)They have the same absolute temperature.
A)The moles of N2O and C3H8 are equal.
B)They have the same density.
C)They have the same number of molecules.
D)They have the same number of atoms.
E)They have the same absolute temperature.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
36
Which conditions of P,T,and n,respectively,are most ideal?
A)low P,low T,low n
B)high P,low T,high n
C)low P,high T,high n
D)low P,high T,low n
E)high P,high T,high n
A)low P,low T,low n
B)high P,low T,high n
C)low P,high T,high n
D)low P,high T,low n
E)high P,high T,high n

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
37
A 23.6 -L sample of nitrogen at 4.45 atm and 23°C is simultaneously expanded to 50.8 L and heated to 37°C.What is the new pressure of the gas?
A)3.33 atm
B)2.17 atm
C)167 atm
D)257 atm
E)1.97 atm
A)3.33 atm
B)2.17 atm
C)167 atm
D)257 atm
E)1.97 atm

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
38
The behavior of PH3(g)is most likely to approach ideal behavior at
A)1.0 atm and 100°C.
B)0.10 atm and -100°C.
C)10 atm and 100°C.
D)0.10 atm and 100°C.
E)1.0 atm and 0°C.
A)1.0 atm and 100°C.
B)0.10 atm and -100°C.
C)10 atm and 100°C.
D)0.10 atm and 100°C.
E)1.0 atm and 0°C.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
39
The volume of a sample of gas measured at 35.0°C and 1.00 atm pressure is 2.00 L.What must the final temperature be in order for the gas to have a final volume of 3.00 L at 1.00 atm pressure?
A)189.0°C
B)52.5°C
C)-220.5°C
D)23.3°C
E)-67.7°C
A)189.0°C
B)52.5°C
C)-220.5°C
D)23.3°C
E)-67.7°C

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
40
Equal volumes of propane,C3H8,and carbon monoxide,CO,at the same temperature and pressure have the same
A)chemical properties.
B)number of atoms.
C)average molecular speed.
D)density.
E)number of molecules.
A)chemical properties.
B)number of atoms.
C)average molecular speed.
D)density.
E)number of molecules.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
41
A 3.74-L cylinder contains 4.17 g of methane,CH4,at a pressure of 3020 mmHg.What is the temperature of the gas?
A)696°C
B)423°C
C)969°C
D)2900°C
E)129°C
A)696°C
B)423°C
C)969°C
D)2900°C
E)129°C

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following statements is incorrect regarding a 128-g sample of gaseous sulfur dioxide at 0°C and 760 mmHg pressure?
A)The density of the gas is 2.86 g/L.
B)There are 2 × 6.02 × 1023 atoms of oxygen present.
C)There are 2 mol of gas present.
D)The molar mass of the gas is 64 g/mol.
E)The volume of the gas is 44.8 L.
A)The density of the gas is 2.86 g/L.
B)There are 2 × 6.02 × 1023 atoms of oxygen present.
C)There are 2 mol of gas present.
D)The molar mass of the gas is 64 g/mol.
E)The volume of the gas is 44.8 L.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following graphs does not correctly describe the ideal gas law?
A)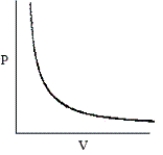
B)They all correctly represent the ideal gas law.
C)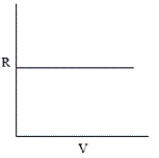
D)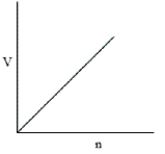
E)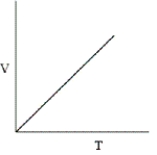
A)

B)They all correctly represent the ideal gas law.
C)

D)

E)


Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
44
The density of ethane,C2H6 (30.1 g/mol),at 25°C and 1.13 atm pressure is
A)1.39 g/L.
B)16.6 g/L.
C)1.34 g/L.
D)0.719 g/L.
E)0.136 g/L.
A)1.39 g/L.
B)16.6 g/L.
C)1.34 g/L.
D)0.719 g/L.
E)0.136 g/L.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following gases has the greatest density at 2.5 atm and 25°C?
A)C4H8
B)SO2
C)N2O
D)O2
E)NF3
A)C4H8
B)SO2
C)N2O
D)O2
E)NF3

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
46
A 22.4 L high pressure reaction vessel is charged with 0.5020 mol of iron powder and 1.39 atm of oxygen gas at standard temperature.On heating,the iron and oxygen react according to the balanced reaction below.
4Fe(s)+ 3O2(g)→ 2Fe2O3(s)
After the reaction vessel is cooled,and assuming the reaction goes to completion,what pressure of oxygen remains?
A)1 atm
B)1.385 atm
C)0.715 atm
D)0.376 atm
E)0.251 atm
4Fe(s)+ 3O2(g)→ 2Fe2O3(s)
After the reaction vessel is cooled,and assuming the reaction goes to completion,what pressure of oxygen remains?
A)1 atm
B)1.385 atm
C)0.715 atm
D)0.376 atm
E)0.251 atm

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
47
The density of O2 gas at 16°C and 1.27 atm is
A)31 g/L.
B)28.4 g/L.
C)1.71 g/L.
D)88.7 g/mL.
E)0.584 g/L.
A)31 g/L.
B)28.4 g/L.
C)1.71 g/L.
D)88.7 g/mL.
E)0.584 g/L.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
48
At 25°C and 449 mmHg,an unknown pure gas has a density of 0.676 g/L.Which of the following gases could be the unknown gas?
A)Cl2
B)C3H6
C)Cl2O
D)Ne
E)N2
A)Cl2
B)C3H6
C)Cl2O
D)Ne
E)N2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
49
The number of molecules in 1.0 L of air at 0°C and 1.0 atm pressure is
A)between 1020 and 1021.
B)between 1023 and 6 × 1023.
C)between 1022 and 1023.
D)less than 1020.
E)between 1021 and 1022.
A)between 1020 and 1021.
B)between 1023 and 6 × 1023.
C)between 1022 and 1023.
D)less than 1020.
E)between 1021 and 1022.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
50
How many moles of gas are in a gas sample occupying 0.816 L at 127 mmHg and 22°C?
A)178 mol
B)4.28 mol
C)57.4 mol
D)0.00563 mol
E)0.000462 mol
A)178 mol
B)4.28 mol
C)57.4 mol
D)0.00563 mol
E)0.000462 mol

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
51
The density of a gas is 0.803 g/L at STP.What is its molar mass?
A)5.0 g/mol
B)24.1 g/mol
C)27.9 g/mol
D)22.4 g/mol
E)18.0 g/mol
A)5.0 g/mol
B)24.1 g/mol
C)27.9 g/mol
D)22.4 g/mol
E)18.0 g/mol

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
52
An unknown gaseous hydrocarbon consists of 85.63% carbon by mass.A 0.959-g sample of the gas occupies a volume of 0.51 L at STP.What is the identity of the gas?
A)C3H6
B)C4H8
C)C5H10
D)CH2
E)C2H4
A)C3H6
B)C4H8
C)C5H10
D)CH2
E)C2H4

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
53
What is the pressure of a 34.8-L gas sample containing 7.45 mol of gas at 19.9°C? (R = 0.0821 L • atm/(K • mol),1 atm = 760 torr)
A)2.66 × 102 mmHg
B)6.77× 10-3 mmHg
C)5.14 mmHg
D)3.91 × 103 mmHg
E)1.48 × 102 mmHg
A)2.66 × 102 mmHg
B)6.77× 10-3 mmHg
C)5.14 mmHg
D)3.91 × 103 mmHg
E)1.48 × 102 mmHg

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
54
For an ideal gas,which of the following statements is true?
A)P is inversely proportional to n at constant V and T.
B)V is inversely proportional to T at constant n and P.
C)V is inversely proportional to n at constant P and T.
D)n is inversely proportional to T at constant P and V.
E)P is inversely proportional to T at constant n and V.
A)P is inversely proportional to n at constant V and T.
B)V is inversely proportional to T at constant n and P.
C)V is inversely proportional to n at constant P and T.
D)n is inversely proportional to T at constant P and V.
E)P is inversely proportional to T at constant n and V.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
55
A mixture consisting of 0.140 mol N2,0.037 mol O2,0.104 mol CH4,and an unknown amount of CO2 occupies a volume of 8.48 L at 27°C and 1.06 atm pressure.How many moles of CO2 are there in this sample?
A)0.719 mol
B)0.0839 mol
C)2.45 mol
D)3.77 mol
E)0.364 mol
A)0.719 mol
B)0.0839 mol
C)2.45 mol
D)3.77 mol
E)0.364 mol

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
56
The volume of 1 mol of nitrogen
A)is lower than that of 1mol ammonia at high pressures.
B)is decreased by decreasing the pressure of the gas.
C)has the value of 22.4 L at 0°C and 1.00 atm.
D)is decreased by increasing its kinetic energy.
E)is increased by decreasing the temperature.
A)is lower than that of 1mol ammonia at high pressures.
B)is decreased by decreasing the pressure of the gas.
C)has the value of 22.4 L at 0°C and 1.00 atm.
D)is decreased by increasing its kinetic energy.
E)is increased by decreasing the temperature.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
57
How many moles of gas are in a gas sample occupying 1.40 L at 553 mmHg and 294 K?
A)32.1 mol
B)0.0422 mol
C)23.7 mol
D)0.00346 mol
E)2.63 mol
A)32.1 mol
B)0.0422 mol
C)23.7 mol
D)0.00346 mol
E)2.63 mol

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
58
The following gases are stored in identical flexible containers at 25°C and 1.00 atm pressure.Order the gases from highest to lowest density.
1)200 g propane,C3H8
2)100 g carbon dioxide,CO2
3)50 g nitrous oxide,N2O
A)1 ≈ 2 ≈ 3
B)3 > 2 > 1
C)1 > 2 > 3
D)2 > 1> 3
E)1 > 2 ≈ 3
1)200 g propane,C3H8
2)100 g carbon dioxide,CO2
3)50 g nitrous oxide,N2O
A)1 ≈ 2 ≈ 3
B)3 > 2 > 1
C)1 > 2 > 3
D)2 > 1> 3
E)1 > 2 ≈ 3

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
59
An excess of sodium hydroxide is treated with 2.9 L of dry hydrogen chloride gas measured at STP.What is the mass of sodium chloride is formed?
A)1.45g
B)15.1 g
C)7.6 g
D)15 g
E)169 g
A)1.45g
B)15.1 g
C)7.6 g
D)15 g
E)169 g

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following samples has the fewest moles of gas?
A)1.00 L of CH4 at STP
B)1.00 L of Ar at -10.0°C and 1.00 atm
C)1.00 L of NH3 at STP
D)1.00 L of H2 at 0.0°C and 1.54 atm
E)1.00 L of HCl at 20°C and 1.00 atm
A)1.00 L of CH4 at STP
B)1.00 L of Ar at -10.0°C and 1.00 atm
C)1.00 L of NH3 at STP
D)1.00 L of H2 at 0.0°C and 1.54 atm
E)1.00 L of HCl at 20°C and 1.00 atm

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
61
The following equation represents the oxidation of ammonia,NH3.
4NH3(g)+ 5O2(g)→ 4NO(g)+ 6H2O(g)
At the same temperature and pressure,what is the maximum volume of nitrogen monoxide that can be obtained from 7.55 × 102 L of ammonia and 7.55 × 102 L of oxygen?
A)1.7 × 103 L
B)3.36 × 102 L
C)6.04 × 102 L
D)1.51 × 103 L
E)7.55 × 102 L
4NH3(g)+ 5O2(g)→ 4NO(g)+ 6H2O(g)
At the same temperature and pressure,what is the maximum volume of nitrogen monoxide that can be obtained from 7.55 × 102 L of ammonia and 7.55 × 102 L of oxygen?
A)1.7 × 103 L
B)3.36 × 102 L
C)6.04 × 102 L
D)1.51 × 103 L
E)7.55 × 102 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
62
A 28.3-g mixture of oxygen and argon is found to occupy a volume of 17.2 L when measured at 882.7 mmHg and 39.3oC.What is the partial pressure of oxygen in this mixture?
A)369 mmHg
B)441 mmHg
C)512 mmHg
D)418 mmHg
E)464 mmHg
A)369 mmHg
B)441 mmHg
C)512 mmHg
D)418 mmHg
E)464 mmHg

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
63
In a mixture of helium and chlorine,occupying a volume of 12.8 L at 605.6 mmHg and 21.6oC,it is found that the partial pressure of chlorine is 143 mmHg.What is the total mass of the sample?
A)31.6 g
B)0.4 g
C)8.37 g
D)1.28 g
E)7.09 g
A)31.6 g
B)0.4 g
C)8.37 g
D)1.28 g
E)7.09 g

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
64
Which statement is inconsistent with the kinetic theory of an ideal gas?
A)Most of the volume occupied by a gas is empty space.
B)The forces of repulsion between gas molecules are very weak or negligible.
C)Gas molecules move in a straight line between collisions.
D)The average kinetic energy of a gas is proportional to the absolute temperature.
E)The collisions between gas molecules are inelastic.
A)Most of the volume occupied by a gas is empty space.
B)The forces of repulsion between gas molecules are very weak or negligible.
C)Gas molecules move in a straight line between collisions.
D)The average kinetic energy of a gas is proportional to the absolute temperature.
E)The collisions between gas molecules are inelastic.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
65
A sample of oxygen is collected over water at a total pressure of 678.4 mmHg at 25°C.The vapor pressure of water at 25°C is 23.8 mmHg.The partial pressure of the O2 is
A)0.9239 atm.
B)0.9161 atm.
C)0.8926 atm.
D)1.092 atm.
E)0.8613 atm.
A)0.9239 atm.
B)0.9161 atm.
C)0.8926 atm.
D)1.092 atm.
E)0.8613 atm.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following is included as a postulate in the kinetic molecular theory of an ideal gas?
A)Collisions between molecules are all elastic.
B)All molecules move randomly in zigzag directions.
C)The distance between gas molecules is small compared with the size of the molecule.
D)All the molecules have the same velocity.
E)In an average collision between molecules,both molecules have the same kinetic energy.
A)Collisions between molecules are all elastic.
B)All molecules move randomly in zigzag directions.
C)The distance between gas molecules is small compared with the size of the molecule.
D)All the molecules have the same velocity.
E)In an average collision between molecules,both molecules have the same kinetic energy.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
67
What volume of ammonia gas,measured at 547.9 mmHg and 27.6oC,is required to produce 8.98 g of ammonium sulfate according to the following balanced chemical equation?
2NH3(g)+ H2SO4(aq)→ (NH4)2SO4(s)
A)0.000992 L
B)0.00397 L
C)1.16 L
D)18 L
E)4.65 L
2NH3(g)+ H2SO4(aq)→ (NH4)2SO4(s)
A)0.000992 L
B)0.00397 L
C)1.16 L
D)18 L
E)4.65 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
68
A 15.6-g mixture of nitrogen and carbon dioxide is found to occupy a volume of 9.9 L when measured at 940.2 mmHg and 54.9°C.What is the mole fraction of carbon dioxide in this mixture?
A)0.486
B)0.5
C)0.513
D)0.597
E)0.402
A)0.486
B)0.5
C)0.513
D)0.597
E)0.402

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
69
A small amount wet of hydrogen gas (H2)can be prepared by the reaction of zinc with excess hydrochloric acid and trapping the gas produced in an inverted tube initially filled with water.If the total pressure of the gas in the collection tube is 757.9 mmHg at 25°C,what is the partial pressure of the hydrogen? The vapor pressure of water is 23.8 mmHg.
A)734.1 mmHg
B)781.7 mmHg
C)757.9 mmHg
D)32.8 mmHg
E)47.7 mmHg
A)734.1 mmHg
B)781.7 mmHg
C)757.9 mmHg
D)32.8 mmHg
E)47.7 mmHg

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
70
A vessel with a volume of 12.4 L contains 2.80 g of nitrogen gas,0.403 g of hydrogen gas,and 79.9 g of argon gas.At 25°C,what is the pressure in the vessel?
A)164 atm
B)256.2 atm
C)4.54 atm
D)0.380 atm
E)2.27 atm
A)164 atm
B)256.2 atm
C)4.54 atm
D)0.380 atm
E)2.27 atm

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
71
The following equation represents the partial combustion of methane,CH4.
2CH4(g)+ 3O2(g)→ 2CO(g)+ 4H2O(g)
At constant temperature and pressure,what is the maximum volume of carbon monoxide that can be obtained from 6.62 × 102 L of methane and 3.31 × 102 L of oxygen?
A)9.93 × 102 L
B)6.62 × 102 L
C)2.32 × 103 L
D)2.21 × 102 L
E)1.32 × 103 L
2CH4(g)+ 3O2(g)→ 2CO(g)+ 4H2O(g)
At constant temperature and pressure,what is the maximum volume of carbon monoxide that can be obtained from 6.62 × 102 L of methane and 3.31 × 102 L of oxygen?
A)9.93 × 102 L
B)6.62 × 102 L
C)2.32 × 103 L
D)2.21 × 102 L
E)1.32 × 103 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
72
A high temperature reaction vessel is charged with 0.5080 mol of iron powder and 42.1 L of oxygen gas at standard temperature at pressure.On heating,the iron and oxygen react according to the balanced reaction below.
4Fe(s)+ 3O2(g)→ 2Fe2O3(s)
After the reaction vessel is cooled,and assuming the reaction goes to completion,what volume of oxygen remains?
A)33.5 L
B)42.1 L
C)26.9 L
D)8.53 L
E)0.254 L
4Fe(s)+ 3O2(g)→ 2Fe2O3(s)
After the reaction vessel is cooled,and assuming the reaction goes to completion,what volume of oxygen remains?
A)33.5 L
B)42.1 L
C)26.9 L
D)8.53 L
E)0.254 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
73
A sample of hydrogen was collected by water displacement at 23.0°C and an atmospheric pressure of 735 mmHg.Its volume is 568 mL.After water vapor is removed,what volume would the hydrogen occupy at the same conditions of pressure and temperature? (The vapor pressure of water at 23.0°C is 21 mmHg.)
A)509 mL
B)539 mL
C)552 mL
D)568 mL
E)585 mL
A)509 mL
B)539 mL
C)552 mL
D)568 mL
E)585 mL

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
74
What is the volume occupied by a mixture of 0.774 mol of N2 and 0.774 mol of O2 gases at 1.04 atm and 25.6°C?
A)1.1 × 103 L
B)36.5 L
C)1.56 L
D)3.12 L
E)18.2 L
A)1.1 × 103 L
B)36.5 L
C)1.56 L
D)3.12 L
E)18.2 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
75
In which of the following reactions will the pressure increase upon completion of the reaction at constant temperature?
A)C(s)+ O2(g)→ CO2(g)
B)2NO(g)+ O2(g)→ 2NO2(g)
C)C2H6O(l)+ 3O2(g)→ 2CO2(g)+ 3H2O(l)
D)4NH3(g)+ 5O2(g)→ 4NO(g)+ 6H2O(g)
E)Cl2(g)+ 3F2(g)→ 2ClF3(g)
A)C(s)+ O2(g)→ CO2(g)
B)2NO(g)+ O2(g)→ 2NO2(g)
C)C2H6O(l)+ 3O2(g)→ 2CO2(g)+ 3H2O(l)
D)4NH3(g)+ 5O2(g)→ 4NO(g)+ 6H2O(g)
E)Cl2(g)+ 3F2(g)→ 2ClF3(g)

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
76
Calcium nitrate will react with ammonium chloride at slightly elevated temperatures,as represented in the equation below.
Ca(NO3)2(s)+ 2NH4Cl(s)→ 2N2O(g)+ CaCl2(s)+ 4H2O(g)
What is the maximum volume of N2O at STP that could be produced using a 5.20-mol sample of each reactant?
A)1.42 × 103 L
B)233 L
C)8.58 × 10-3 L
D)116 L
E)22.4 L
Ca(NO3)2(s)+ 2NH4Cl(s)→ 2N2O(g)+ CaCl2(s)+ 4H2O(g)
What is the maximum volume of N2O at STP that could be produced using a 5.20-mol sample of each reactant?
A)1.42 × 103 L
B)233 L
C)8.58 × 10-3 L
D)116 L
E)22.4 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
77
The partial pressures of CH4,N2,and O2 in a sample of gas were found to be 183 mmHg,443 mmHg,and 693 mmHg,respectively.What is the mole fraction of nitrogen?
A)21.7
B)0.912
C)0.525
D)0.410
E)0.336
A)21.7
B)0.912
C)0.525
D)0.410
E)0.336

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
78
What is the partial pressure of carbon dioxide in a container that contains 3.63 mol of oxygen,1.49 mol of nitrogen,and 4.49 mol of carbon dioxide when the total pressure is 871 mmHg?
A)329 mmHg
B)763 mmHg
C)135 mmHg
D)406 mmHg
E)871 mmHg
A)329 mmHg
B)763 mmHg
C)135 mmHg
D)406 mmHg
E)871 mmHg

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
79
What is the total volume of gases produced at 1092 K and 1.00 atm pressure when 320 g of ammonium nitrite undergoes the following decomposition reaction?
NH4NO2(s)→ N2(g)+ 2H2O(g)
A)4 × 22.4 L
B)22.4 L
C)20 × 22.4 L
D)5 × 22.4 L
E)60 × 22.4 L
NH4NO2(s)→ N2(g)+ 2H2O(g)
A)4 × 22.4 L
B)22.4 L
C)20 × 22.4 L
D)5 × 22.4 L
E)60 × 22.4 L

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
80
If 636.0 mL of nitrogen gas,measured at 488.9 mmHg and 22.3oC,reacts with excess iodine according to the following reaction,what mass of nitrogen triiodide is produced?
N2(g)+ 3I2(s)→ 2NI3(s)
A)0.472 g
B)6.66 g
C)3.33 g
D)13.3 g
E)176 g
N2(g)+ 3I2(s)→ 2NI3(s)
A)0.472 g
B)6.66 g
C)3.33 g
D)13.3 g
E)176 g

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck


