Deck 8: How Water Behaves
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/140
Play
Full screen (f)
Deck 8: How Water Behaves
1
The formation of hydrogen bonds between water molecules ________.
A)leads to the formation of ice
B)releases heat energy
C)increases the amount of molecular vibration
D)causes ice to melt and form water
E)makes molecules move farther apart
A)leads to the formation of ice
B)releases heat energy
C)increases the amount of molecular vibration
D)causes ice to melt and form water
E)makes molecules move farther apart
releases heat energy
2
As an ice cube floating in a glass of water melts, what happens to the water level?
A)The water level rises to the point where the ice floats above the water.
B)As the ice melts, the water level does not change-the melting ice "caves in" and exactly fills the open spaces.
C)The melting ice "caves in" and fills the open spaces-therefore the water level is lower after the ice melts.
A)The water level rises to the point where the ice floats above the water.
B)As the ice melts, the water level does not change-the melting ice "caves in" and exactly fills the open spaces.
C)The melting ice "caves in" and fills the open spaces-therefore the water level is lower after the ice melts.
As the ice melts, the water level does not change-the melting ice "caves in" and exactly fills the open spaces.
3
Why does water expand when it goes from a liquid to a solid?
A)The water molecules become aligned in a way that creates extra volume.
B)The liquid has a higher density than the ice.
C)Ice molecules are bigger than water molecules.
D)The liquid water molecules are bigger and shrink when cooled.
E)none of the above
A)The water molecules become aligned in a way that creates extra volume.
B)The liquid has a higher density than the ice.
C)Ice molecules are bigger than water molecules.
D)The liquid water molecules are bigger and shrink when cooled.
E)none of the above
The water molecules become aligned in a way that creates extra volume.
4
If you keep a mixture of water and ice at 0°C what will happen?
A)Nothing, the ice and water will stay the same.
B)The water will freeze and become ice.
C)The ice will melt and become water.
D)The water will dissolve the ice.
E)The ice will absorb the water.
A)Nothing, the ice and water will stay the same.
B)The water will freeze and become ice.
C)The ice will melt and become water.
D)The water will dissolve the ice.
E)The ice will absorb the water.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
5
How does a solute such as salt or sugar affect the melting and freezing of water?
A)Solutes slow the rate of ice formation.
B)Solutes increase the rate of ice formation.
C)Solutes slow the rate of liquid water release from ice.
D)Solutes increase the rate of liquid water release from ice.
E)none of the above
A)Solutes slow the rate of ice formation.
B)Solutes increase the rate of ice formation.
C)Solutes slow the rate of liquid water release from ice.
D)Solutes increase the rate of liquid water release from ice.
E)none of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
6
Heat is absorbed by a substance when that substance ________.
A)freezes
B)evaporates
C)condenses
D)cools
E)A and C
A)freezes
B)evaporates
C)condenses
D)cools
E)A and C

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
7
How does the combined volume of the billions and billions of hexagonal open spaces in the structures of ice crystals in a piece of ice compare to the portion of ice that floats above the water line?
A)They are the same.
B)The volume of the open spaces is greater than the volume that extends above water.
C)The volume of the open space is less than the volume that extends above water.
D)The volumes are not related to each other.
A)They are the same.
B)The volume of the open spaces is greater than the volume that extends above water.
C)The volume of the open space is less than the volume that extends above water.
D)The volumes are not related to each other.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
8
If you add heat energy to water molecules in the form of ice, where does the energy go?
A)The energy is used to break hydrogen bonds.
B)Energy is neither created nor destroyed.
C)The energy is used to make hydrogen bonds.
D)Energy causes the molecules to arrange in regular arrays.
E)none of the above
A)The energy is used to break hydrogen bonds.
B)Energy is neither created nor destroyed.
C)The energy is used to make hydrogen bonds.
D)Energy causes the molecules to arrange in regular arrays.
E)none of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
9
Water is most dense at ________.
A)-4°C
B)0°C
C)+4°C
D)+100°C
A)-4°C
B)0°C
C)+4°C
D)+100°C

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements best describes the usual properties of most compounds besides water?
A)The density of a material increases when it solidifies.
B)The volume of a material increases when it solidifies.
C)The mass of a material increases when it solidifies.
D)The molecules become more closely packed when they liquify.
E)none of the above
A)The density of a material increases when it solidifies.
B)The volume of a material increases when it solidifies.
C)The mass of a material increases when it solidifies.
D)The molecules become more closely packed when they liquify.
E)none of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
11
What happens when pressure is applied to ice crystals?
A)The regular arrangement of the ice is disrupted and the ice melts.
B)The temperature rises and the ice melts.
C)The regular arrangement of ice causes it to harden.
D)The freezing temperature of the molecules decreases.
E)The volume occupied by the material increases.
A)The regular arrangement of the ice is disrupted and the ice melts.
B)The temperature rises and the ice melts.
C)The regular arrangement of ice causes it to harden.
D)The freezing temperature of the molecules decreases.
E)The volume occupied by the material increases.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
12
At the molecular level, when a piece of ice is slowly melting, water molecules are being ________.
A)adsorbed by the ice to form solid water
B)released by the ice to form liquid water
C)adsorbed by the ice to form liquid water
D)released by the ice to form solid water
E)both A and B
A)adsorbed by the ice to form solid water
B)released by the ice to form liquid water
C)adsorbed by the ice to form liquid water
D)released by the ice to form solid water
E)both A and B

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements does not describe a property of water?
A)You are made mostly of water.
B)It is the only molecule that is found in all three phases (solid, liquid, gas)in large quantities on the planet.
C)Water is very resistant to a change in temperature.
D)Many of the properties of water are a direct consequence of the intermolecular attractions between water molecules.
E)The hydrogens of a water molecule have a partial negative charge.
A)You are made mostly of water.
B)It is the only molecule that is found in all three phases (solid, liquid, gas)in large quantities on the planet.
C)Water is very resistant to a change in temperature.
D)Many of the properties of water are a direct consequence of the intermolecular attractions between water molecules.
E)The hydrogens of a water molecule have a partial negative charge.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
14
Heat is given off by a substance when that substance ________.
A)evaporates
B)freezes
C)melts
D)boils
E)C and D
A)evaporates
B)freezes
C)melts
D)boils
E)C and D

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
15
For an ice/water mixture at 0°C, which is true?
A)The rate at which ice melts is faster.
B)The rate at which water freezes is faster
C)The rate at which ice melts and freezes are both equal.
D)Ice always freezes at 0°C.
E)Ice always melts at 0°C.
A)The rate at which ice melts is faster.
B)The rate at which water freezes is faster
C)The rate at which ice melts and freezes are both equal.
D)Ice always freezes at 0°C.
E)Ice always melts at 0°C.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
16
What is found within the extra volume that arises as water expands upon freezing?
A)nothing
B)other water molecules
C)air
D)dissolved oxygen
E)the atoms have expanded
A)nothing
B)other water molecules
C)air
D)dissolved oxygen
E)the atoms have expanded

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
17
Ice is slippery because ________.
A)air molecules have been excluded
B)of greater kinetic energy at the surface
C)of its relatively high purity
D)surface crystals fall apart
E)of its tight internal and external structuring
A)air molecules have been excluded
B)of greater kinetic energy at the surface
C)of its relatively high purity
D)surface crystals fall apart
E)of its tight internal and external structuring

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
18
It is important to protect water pipes in your home from freezing because ________.
A)freezing water will scrape the lining from the insides of the pipes
B)water expands more than the pipe material, which will fracture the pipes if water in them freezes
C)blocked water behind the frozen ice will build up pressure and will burst the pipes
D)the ice will promote serious erosion of the pipe material
E)all of the above
A)freezing water will scrape the lining from the insides of the pipes
B)water expands more than the pipe material, which will fracture the pipes if water in them freezes
C)blocked water behind the frozen ice will build up pressure and will burst the pipes
D)the ice will promote serious erosion of the pipe material
E)all of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
19
Why do ice skates work?
A)The pressure of the blades on the ice creates a temporary water layer.
B)The pressure of the blades cuts a hole in the ice and water flows in.
C)The blades are treated with a chemical that melts the ice temporarily, which creates a water layer.
D)Ice is naturally slippery.
A)The pressure of the blades on the ice creates a temporary water layer.
B)The pressure of the blades cuts a hole in the ice and water flows in.
C)The blades are treated with a chemical that melts the ice temporarily, which creates a water layer.
D)Ice is naturally slippery.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
20
What happens to a soda can left in the freezer? Why?
A)The sugar and syrup, which separate from the water, settle to the bottom of the can and become solid.
B)The soda inside the can freezes collapsing the can as the solid forms.
C)The soda can puffs out and sometimes the lid pops open because water expands when it freezes.
D)Although the soda inside the can get s very cold, it will not completely freeze because of the high sugar concentration.
A)The sugar and syrup, which separate from the water, settle to the bottom of the can and become solid.
B)The soda inside the can freezes collapsing the can as the solid forms.
C)The soda can puffs out and sometimes the lid pops open because water expands when it freezes.
D)Although the soda inside the can get s very cold, it will not completely freeze because of the high sugar concentration.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
21
Why are polar oceans most fertile in the autumn?
A)The warm summer temperatures allowed for the growth of many microorganisms in the water.
B)As the oxygen rich surface waters cool in the autumn, they sink to the bottom, displacing the layer of decomposing organic matter, forcing it to rise, adding nutrients to the surface of the polar oceans.
C)The salinity of the ocean is at a low point at that time of year.
D)Ocean currents tend to reverse during the autumn, bringing nutrient rich waters up from the equatorial regions.
A)The warm summer temperatures allowed for the growth of many microorganisms in the water.
B)As the oxygen rich surface waters cool in the autumn, they sink to the bottom, displacing the layer of decomposing organic matter, forcing it to rise, adding nutrients to the surface of the polar oceans.
C)The salinity of the ocean is at a low point at that time of year.
D)Ocean currents tend to reverse during the autumn, bringing nutrient rich waters up from the equatorial regions.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
22
Why does water not freeze at 0°C when either ions or molecules other than 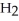 O are present?
O are present?
A)Molecules or ions dissolved in the water give the water molecules something else to easily bond with, thus allowing the solution to freeze well above 0°C.
B)Molecules or ions dissolved in the water increase the number of water molecules at the solid-liquid interface, thus allowing for the rate of ice formation to increase at a temperature above 0°C.
C)When molecules or ions are dissolved in the water, these solute molecules both take up space and inhibit the formation of the solid phase, thus lowering the freezing point temperature below 0°C.
D)Molecules or ions dissolved in the water will sometimes raise the freezing point above 0°C and other times lower it below 0°C depending on the nature of these solute particles.
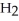 O are present?
O are present?A)Molecules or ions dissolved in the water give the water molecules something else to easily bond with, thus allowing the solution to freeze well above 0°C.
B)Molecules or ions dissolved in the water increase the number of water molecules at the solid-liquid interface, thus allowing for the rate of ice formation to increase at a temperature above 0°C.
C)When molecules or ions are dissolved in the water, these solute molecules both take up space and inhibit the formation of the solid phase, thus lowering the freezing point temperature below 0°C.
D)Molecules or ions dissolved in the water will sometimes raise the freezing point above 0°C and other times lower it below 0°C depending on the nature of these solute particles.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
23
Why does adding heat to ice-water disfavor the rate of ice formation?
A)Heat increases the vibration of the water molecules, which breaks the hydrogen bonds.
B)When heat is added, the water molecules are moving too fast to form hydrogen bonds, which gives it the structure that we call ice.
C)Adding heat favors the rate of melting, as more hydrogen bonds are broken than formed by the ice in the ice-water.
D)all the above
A)Heat increases the vibration of the water molecules, which breaks the hydrogen bonds.
B)When heat is added, the water molecules are moving too fast to form hydrogen bonds, which gives it the structure that we call ice.
C)Adding heat favors the rate of melting, as more hydrogen bonds are broken than formed by the ice in the ice-water.
D)all the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
24
Describe what happens to an ice cube and some water at 0°C if you increase the rate of water formation?
A)The ice cube melts.
B)Nothing, ice and water at 0°C is stable indefinitely.
C)The ice cube increases in size.
D)The rate of ice formation also increases.
E)none of the above
A)The ice cube melts.
B)Nothing, ice and water at 0°C is stable indefinitely.
C)The ice cube increases in size.
D)The rate of ice formation also increases.
E)none of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
25
Why is calcium chloride, 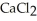 , more effective at melting ice than sodium chloride, NaCl?
, more effective at melting ice than sodium chloride, NaCl?
A)Calcium ions are both larger and more highly charged and thus more effective in breaking the ice crystal structure.
B)Calcium ions are smaller than sodium ions and thus more able to get in the way of the water molecules attempting to form the solid state lattice.
C)Calcium chloride produces three ion particles while sodium chloride produces only two. Greater numbers of ions are more effective at decreasing the number of water molecules entering the solid phase.
D)Sodium ion is a natural water softener and therefore not as effective in inhibiting the formation of the solid state of water from the liquid.
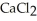 , more effective at melting ice than sodium chloride, NaCl?
, more effective at melting ice than sodium chloride, NaCl?A)Calcium ions are both larger and more highly charged and thus more effective in breaking the ice crystal structure.
B)Calcium ions are smaller than sodium ions and thus more able to get in the way of the water molecules attempting to form the solid state lattice.
C)Calcium chloride produces three ion particles while sodium chloride produces only two. Greater numbers of ions are more effective at decreasing the number of water molecules entering the solid phase.
D)Sodium ion is a natural water softener and therefore not as effective in inhibiting the formation of the solid state of water from the liquid.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following describes what happens when you increase the temperature of water?
A)The molecules start to vibrate and move farther apart.
B)The molecules start to vibrate and move closer together.
C)The molecular vibrations start to slow and the molecules move farther apart.
D)The molecular vibrations start to slow and the molecules move closer together.
E)none of the above.
A)The molecules start to vibrate and move farther apart.
B)The molecules start to vibrate and move closer together.
C)The molecular vibrations start to slow and the molecules move farther apart.
D)The molecular vibrations start to slow and the molecules move closer together.
E)none of the above.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
27
Why does ice form at the surface of a body of fresh water instead of at the bottom?
A)Initial freezing requires the presence of air in order to form the ice lattice, thus the surface water will become solid first.
B)Intermolecular forces are greater at the surface allowing the surface molecules to form the solid phase more quickly.
C)Water "near" the freezing point of 0°C is less dense than warmer water, and the colder water will "float" on the warmer water. This allows ice to form at the surface.
D)All of the above are true.
A)Initial freezing requires the presence of air in order to form the ice lattice, thus the surface water will become solid first.
B)Intermolecular forces are greater at the surface allowing the surface molecules to form the solid phase more quickly.
C)Water "near" the freezing point of 0°C is less dense than warmer water, and the colder water will "float" on the warmer water. This allows ice to form at the surface.
D)All of the above are true.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
28
Suppose that water is used in a thermometer instead of mercury. If the temperature is at 4°C and then changes, why can't the thermometer indicate whether the temperature is rising or falling?
A)The water molecules will be a mixture of ice and water, and a change in temperature will only change the proportions of its phases.
B)The water molecules are moving too slowly to be able to indicate a change.
C)Water is most dense at 4°C. The water in a water-filled thermometer at 4°C would expand as the water was cooled or heated.
D)Since water has such a high specific heat capacity, it will take a lot of energy to be able to see a change in the thermometer.
A)The water molecules will be a mixture of ice and water, and a change in temperature will only change the proportions of its phases.
B)The water molecules are moving too slowly to be able to indicate a change.
C)Water is most dense at 4°C. The water in a water-filled thermometer at 4°C would expand as the water was cooled or heated.
D)Since water has such a high specific heat capacity, it will take a lot of energy to be able to see a change in the thermometer.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
29
Which graph most appropriately shows the density of water plotted against temperature? 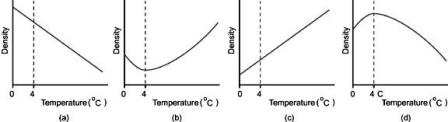
A)graph a
B)graph b
C)graph c
D)graph d

A)graph a
B)graph b
C)graph c
D)graph d

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
30
When you increase the temperature of a substance, the molecules vibrate ________.
A)faster and the molecules move farther apart
B)faster and the molecules more closer together
C)slower and the molecules move farther apart
D)slower and the molecules move closer together
E)none of the above
A)faster and the molecules move farther apart
B)faster and the molecules more closer together
C)slower and the molecules move farther apart
D)slower and the molecules move closer together
E)none of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
31
What normally describes what happens when you decrease the temperature of water?
A)The molecules start to vibrate and move farther apart, increasing the density.
B)The molecules start to vibrate and move closer together, increasing the density.
C)The molecular vibrations start to slow and the molecules move farther apart, decreasing the density.
D)The molecular vibrations start to slow and the molecules move closer together, increasing the density.
E)none of the above
A)The molecules start to vibrate and move farther apart, increasing the density.
B)The molecules start to vibrate and move closer together, increasing the density.
C)The molecular vibrations start to slow and the molecules move farther apart, decreasing the density.
D)The molecular vibrations start to slow and the molecules move closer together, increasing the density.
E)none of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
32
What happens to the freezing temperature of a salt water solution as it becomes more concentrated?
A)As salt water becomes more concentrated, the temperature at which it freezes become lower because fewer water molecules of the liquid phase in contact with the ice.
B)As salt water becomes more concentrated, the temperature at which it freezes become higher because salt ions help to hold together the ice.
C)As salt water becomes more concentrated, the temperature at which it freezes does not change.
D)As salt water becomes more concentrated, the temperature at which it freezes become higher because the molecules are moving faster as they interact with the salt ions.
A)As salt water becomes more concentrated, the temperature at which it freezes become lower because fewer water molecules of the liquid phase in contact with the ice.
B)As salt water becomes more concentrated, the temperature at which it freezes become higher because salt ions help to hold together the ice.
C)As salt water becomes more concentrated, the temperature at which it freezes does not change.
D)As salt water becomes more concentrated, the temperature at which it freezes become higher because the molecules are moving faster as they interact with the salt ions.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
33
Unlike fresh water, ocean water contracts as it is cooled all the way down to its freezing point, which is about -18°C. Why?
A)As the water cools, it becomes more dense and then it sinks. The pressure from the weight of the ocean exerted on the ice below the surface of water causes it to be more dense.
B)macrocrystals instead of microcrystals are formed during the freezing of ocean water.
C)As the water cools, the salt ions in the ocean water pull the molecules closer together.
D)The presence of a solute disrupts the rate of ice microcrystal formation.
A)As the water cools, it becomes more dense and then it sinks. The pressure from the weight of the ocean exerted on the ice below the surface of water causes it to be more dense.
B)macrocrystals instead of microcrystals are formed during the freezing of ocean water.
C)As the water cools, the salt ions in the ocean water pull the molecules closer together.
D)The presence of a solute disrupts the rate of ice microcrystal formation.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
34
Is the density of near-freezing water, which contains microscopic ice crystals, greater or less than the density of liquid water containing no microscopic ice crystals?
A)less dense
B)more dense
C)It's the same since it's all water.
D)It's not possible to determine the density of any substance which exists in two phases at once.
A)less dense
B)more dense
C)It's the same since it's all water.
D)It's not possible to determine the density of any substance which exists in two phases at once.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
35
Consider a lake that is uniformly 10°C. What happens to the oxygen-rich surface water as it cools down to  ?
?
A)The oxygen gets squeezed out as the microcrystals form.
B)Its rate of evaporation decreases which has the effect of increasing the oxygen concentration.
C)It grows less dense and remains at the surface.
D)It grows more dense and sinks towards the bottom.
 ?
?A)The oxygen gets squeezed out as the microcrystals form.
B)Its rate of evaporation decreases which has the effect of increasing the oxygen concentration.
C)It grows less dense and remains at the surface.
D)It grows more dense and sinks towards the bottom.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following statements describes what happens when a given mass of a liquid starts to expand?
A)Its density decreases.
B)Its density increases.
C)Its density does not change.
D)Its mass increases.
E)Its volume decreases.
A)Its density decreases.
B)Its density increases.
C)Its density does not change.
D)Its mass increases.
E)Its volume decreases.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
37
Why does a lake freeze from the top down?
A)As cold water cools towards freezing it gets less dense.
B)The cold air is at the top of the lake.
C)Cold water sinks, warm water rises.
D)Warm water sinks, cold water rises.
E)none of the above
A)As cold water cools towards freezing it gets less dense.
B)The cold air is at the top of the lake.
C)Cold water sinks, warm water rises.
D)Warm water sinks, cold water rises.
E)none of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
38
Ice floats in room temperature water, but does it float in boiling water? Why or why not?
A)As liquid water heats up and boils, it will become less dense, therefore the ice will sink.
B)As water cools, it becomes more dense. When it freezes however, it becomes less dense as it forms ice crystals. Therefore, it will sink in boiling water, but float in water at room temperature.
C)It is impossible to have ice in boiling water.
D)If boiling water is less dense than ice, the ice cube should sink.
A)As liquid water heats up and boils, it will become less dense, therefore the ice will sink.
B)As water cools, it becomes more dense. When it freezes however, it becomes less dense as it forms ice crystals. Therefore, it will sink in boiling water, but float in water at room temperature.
C)It is impossible to have ice in boiling water.
D)If boiling water is less dense than ice, the ice cube should sink.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
39
If you were somehow able to sit at the surface of an ice cube and were able to slow the movement of water molecules from the solid to the liquid phase, what would eventually happen to the ice cube?
A)It would increase in size.
B)It would melt because you are sitting on it and you give off heat.
C)It would melt because you have slowed the rate of water uptake.
D)It would melt because you have increased the rate of water uptake.
E)none of the above
A)It would increase in size.
B)It would melt because you are sitting on it and you give off heat.
C)It would melt because you have slowed the rate of water uptake.
D)It would melt because you have increased the rate of water uptake.
E)none of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
40
Why does liquid water expand slightly when cooled from 4°C to 2°C?
A)The molecules vibrate faster and the molecules move farther apart.
B)The molecules vibrate faster and the molecules move closer together.
C)The molecules vibrate slower and the molecules move farther apart as hydrogen bonds form.
D)The molecules vibrate slower and the molecules move closer together as hydrogen bonds form.
E)none of the above
A)The molecules vibrate faster and the molecules move farther apart.
B)The molecules vibrate faster and the molecules move closer together.
C)The molecules vibrate slower and the molecules move farther apart as hydrogen bonds form.
D)The molecules vibrate slower and the molecules move closer together as hydrogen bonds form.
E)none of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the above liquids has the strongest cohesive forces?
A)a
B)b
C)c
D)d
E)e
A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
42
What is the leading cause of surface tension in water?
A)hydrogen bonding
B)ion-dipole interactions
C)solvation
D)evaporation
A)hydrogen bonding
B)ion-dipole interactions
C)solvation
D)evaporation

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
43
An adhesive force is best described as ________.
A)attraction between two liquid molecules
B)attraction between a liquid molecule and a solute
C)the force that allows for molecular adhesion
D)the force that holds together the nucleus
E)attraction between two different molecules
A)attraction between two liquid molecules
B)attraction between a liquid molecule and a solute
C)the force that allows for molecular adhesion
D)the force that holds together the nucleus
E)attraction between two different molecules

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
44
Dip a paper clip into water and then slowly pull it out. You'll find that for a short distance, the water is brought up with the metal. Are these adhesive or cohesive forces at work?
A)These are the adhesive forces between the water and the metal (two different materials). The type of molecular interactions going on are dipole-induced dipole.
B)These are the adhesive forces between the water molecules. The type of molecular interactions going on are dipole-induced dipole.
C)These are the adhesive forces between the water and the metal (two different materials). The type of molecular interactions going on are dipole-dipole.
D)These are the cohesive forces between the water molecules. Water has such a strong attraction for itself that it will cling to the water on the paper clip until the force of gravity is stronger than its cohesive forces.
A)These are the adhesive forces between the water and the metal (two different materials). The type of molecular interactions going on are dipole-induced dipole.
B)These are the adhesive forces between the water molecules. The type of molecular interactions going on are dipole-induced dipole.
C)These are the adhesive forces between the water and the metal (two different materials). The type of molecular interactions going on are dipole-dipole.
D)These are the cohesive forces between the water molecules. Water has such a strong attraction for itself that it will cling to the water on the paper clip until the force of gravity is stronger than its cohesive forces.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
45
Can a glass be filled to above its brim with water without the water spilling over the edge?
A)Yes, due to the strong cohesive forces between water molecules.
B)Yes, due to the strong adhesive forces between water molecules.
C)Yes, due to the strong cohesive forces of gravity keeping the water in the cup.
D)No, it is just an optical illusion because water magnetizes things.
A)Yes, due to the strong cohesive forces between water molecules.
B)Yes, due to the strong adhesive forces between water molecules.
C)Yes, due to the strong cohesive forces of gravity keeping the water in the cup.
D)No, it is just an optical illusion because water magnetizes things.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
46
A water bug can rest on top of water without getting wet because of ________.
A)surface tension
B)cohesive forces within water
C)its nonpolar exterior
D)all of the above
A)surface tension
B)cohesive forces within water
C)its nonpolar exterior
D)all of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
47
Capillary action causes water to climb up the internal walls of narrow glass tubes. Why does the water not climb so high when the glass tube is wider?
A)The upper surface area is exposed to greater atmospheric pressure.
B)A wider column of water weighs more.
C)The water sticks better to the glass because of the greater surface area of contact.
D)The greater rate of evaporation has the effect of pushing the water back downwards.
A)The upper surface area is exposed to greater atmospheric pressure.
B)A wider column of water weighs more.
C)The water sticks better to the glass because of the greater surface area of contact.
D)The greater rate of evaporation has the effect of pushing the water back downwards.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
48
If all of the liquids pictured above were the same, which setup would contain the capillary with the smallest diameter?
A)a
B)b
C)c
D)d
E)e
A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
49
Why are drops of water shaped like spheres?
A)Water molecules at the surface are pulled into the liquid, making the drop as small as possible.
B)The interatomic forces are strongest for spherical shapes and so the molecules arrange into spheres.
C)Water molecules are composed of spheres and so the bulk material takes on the same shape.
D)Water molecules are special and are the only molecules that form spheres. This is one of water's unique physical properties.
A)Water molecules at the surface are pulled into the liquid, making the drop as small as possible.
B)The interatomic forces are strongest for spherical shapes and so the molecules arrange into spheres.
C)Water molecules are composed of spheres and so the bulk material takes on the same shape.
D)Water molecules are special and are the only molecules that form spheres. This is one of water's unique physical properties.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
50
Would you expect the surface tension of water to increase or decrease with temperature?
A)At warmer temperatures water molecules are moving faster. This increased friction produces a stronger electrical charge, causing them to cohere more strongly, which increases surface tension.
B)At cooler temperatures water molecules are moving slower, which makes it relatively easy for them to cohere to one another. This, in turn, increases the surface tension.
C)At warmer temperatures water molecules are moving faster, which makes it easier for them to bump into and cohere to one another, which increases surface tension.
D)At cooler temperatures, the water molecules adhere more strongly to each other because they condense as the reach freezing point, which increases surface tension.
A)At warmer temperatures water molecules are moving faster. This increased friction produces a stronger electrical charge, causing them to cohere more strongly, which increases surface tension.
B)At cooler temperatures water molecules are moving slower, which makes it relatively easy for them to cohere to one another. This, in turn, increases the surface tension.
C)At warmer temperatures water molecules are moving faster, which makes it easier for them to bump into and cohere to one another, which increases surface tension.
D)At cooler temperatures, the water molecules adhere more strongly to each other because they condense as the reach freezing point, which increases surface tension.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
51
A cohesive force is best described as the ________.
A)attraction between two liquid molecules
B)attraction between a liquid molecule and a solute
C)force that allows for molecular cohesion
D)force that holds together the nucleus
E)attraction between molecules of two different substances
A)attraction between two liquid molecules
B)attraction between a liquid molecule and a solute
C)force that allows for molecular cohesion
D)force that holds together the nucleus
E)attraction between molecules of two different substances

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
52
If all of the capillaries pictured above were the same size diameter, which would contain the liquid with the greatest capillary action?
A)a
B)b
C)c
D)d
E)e
A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
53
Why does a paper clip float when placed on water?
A)It is lighter than water.
B)It is less dense than water.
C)It only floats on dense, cold water.
D)The molecules of water hold together and keep it from sinking.
A)It is lighter than water.
B)It is less dense than water.
C)It only floats on dense, cold water.
D)The molecules of water hold together and keep it from sinking.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following would have the highest surface tension?
A)a molecule with very strong intermolecular forces
B)a molecule with very weak intermolecular forces
C)a molecule with a very small degree of cohesive forces
D)a molecule with very strong adhesive forces
E)a mixture of molecules with strong and weak adhesive forces
A)a molecule with very strong intermolecular forces
B)a molecule with very weak intermolecular forces
C)a molecule with a very small degree of cohesive forces
D)a molecule with very strong adhesive forces
E)a mixture of molecules with strong and weak adhesive forces

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
55
Surface tension is the ________.
A)tendency for molecules at the surface of a liquid to hold together
B)rate at which molecules move into and out of the surface of a liquid
C)tendency for a molecule to pack into spherical shapes
D)tendency for the adhesive forces of a molecule to overcome the cohesive forces
E)none of the above
A)tendency for molecules at the surface of a liquid to hold together
B)rate at which molecules move into and out of the surface of a liquid
C)tendency for a molecule to pack into spherical shapes
D)tendency for the adhesive forces of a molecule to overcome the cohesive forces
E)none of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the above liquids would most likely have the greatest capillary action?
A)a
B)b
C)c
D)d
E)e
A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
57
Why does soap decrease the surface tension of of water?
A)It keeps water molecules away from the surface.
B)It increases the capillary action of the water.
C)It increases the ionic content of the water.
D)It decreases the amount of intermolecular attraction between the water molecules.
E)none of the above
A)It keeps water molecules away from the surface.
B)It increases the capillary action of the water.
C)It increases the ionic content of the water.
D)It decreases the amount of intermolecular attraction between the water molecules.
E)none of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the above liquids would most likely have the least capillary action?
A)a
B)b
C)c
D)d
E)e
A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
59
Mercury forms a convex meniscus with glass and not the concave meniscus formed by water. What does this tell you about the cohesive forces within mercury versus the adhesive forces between mercury and glass? 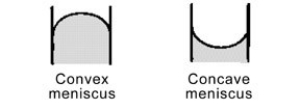
A)Mercury sticks to itself (adhesive forces)better than it sticks to the glass (cohesive forces).
B)The cohesive forces of the glass repel the mercury.
C)Mercury sticks to itself (cohesive forces)better than it sticks to the glass (adhesive forces).
D)The adhesive forces of the glass attract the mercury.

A)Mercury sticks to itself (adhesive forces)better than it sticks to the glass (cohesive forces).
B)The cohesive forces of the glass repel the mercury.
C)Mercury sticks to itself (cohesive forces)better than it sticks to the glass (adhesive forces).
D)The adhesive forces of the glass attract the mercury.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the above liquids has the strongest adhesive forces?
A)a
B)b
C)c
D)d
E)e
A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
61
Why does condensation have a tendency to raise the temperature of a liquid?
A)Water vapor molecules absorb kinetic energy when they slow and form liquid water.
B)Because the liquid water absorbs the water vapor molecules.
C)Condensation leads to slow moving molecules.
D)Hydrogen bonding leads to a increase in the overall amount of kinetic energy in the water vapor.
E)none of the above
A)Water vapor molecules absorb kinetic energy when they slow and form liquid water.
B)Because the liquid water absorbs the water vapor molecules.
C)Condensation leads to slow moving molecules.
D)Hydrogen bonding leads to a increase in the overall amount of kinetic energy in the water vapor.
E)none of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
62
From the diagram above describing the phase of water relative to temperature and pressure, at which point does water exist in all three phases?
A)D
B)B
C)C
D)A
A)D
B)B
C)C
D)A

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
63
From the diagram above describing the phase of xenon relative to temperature and pressure which is more dense: liquid xenon or solid xenon?
A)solid
B)liquid
C)The density of xenon is the same in both the solid and liquid phase.
D)Density information can not be determined from the diagram.
A)solid
B)liquid
C)The density of xenon is the same in both the solid and liquid phase.
D)Density information can not be determined from the diagram.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
64
Why do water drops bead on a freshly waxed surface?
A)The wax, which is nonpolar, pushes the water into a bead shape.
B)The strong cohesive forces between water molecules attract each other and the result is a sphere, which is squashed down into a bead by the force of gravity.
C)Water's cohesive forces between molecules results in a spherical shape and prevents the water droplet from spreading out evenly over the wax.
D)A freshly waxed surface carries a small electrical charge, which repel the outer electrons on the water molecules.
A)The wax, which is nonpolar, pushes the water into a bead shape.
B)The strong cohesive forces between water molecules attract each other and the result is a sphere, which is squashed down into a bead by the force of gravity.
C)Water's cohesive forces between molecules results in a spherical shape and prevents the water droplet from spreading out evenly over the wax.
D)A freshly waxed surface carries a small electrical charge, which repel the outer electrons on the water molecules.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
65
Why does evaporation of a liquid also cool the gas above it?
A)The departing gas molecule takes some kinetic energy with it as it leaves the surface.
B)The overall heat decreases because the air cools.
C)The average energy of the system increases because of the increased disorder in the new gas molecules.
D)The gas molecules need to transfer their kinetic energy to the new slower gas molecule.
E)none of the above
A)The departing gas molecule takes some kinetic energy with it as it leaves the surface.
B)The overall heat decreases because the air cools.
C)The average energy of the system increases because of the increased disorder in the new gas molecules.
D)The gas molecules need to transfer their kinetic energy to the new slower gas molecule.
E)none of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
66
Why does evaporation cool a liquid?
A)The departing gas molecule takes some kinetic energy with it as it leaves the surface.
B)The overall heat decreases because the air cools.
C)The average energy of the system increases because of the increased disorder in the new gas molecules.
D)The gas molecules need to transfer their kinetic energy to the new slower gas molecule.
E)none of the above
A)The departing gas molecule takes some kinetic energy with it as it leaves the surface.
B)The overall heat decreases because the air cools.
C)The average energy of the system increases because of the increased disorder in the new gas molecules.
D)The gas molecules need to transfer their kinetic energy to the new slower gas molecule.
E)none of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
67
Why does sweat cool you off?
A)The water molecules leaving the skin as vapor absorb the heat from your skin.
B)The sweat glands require energy to produce water.
C)The water molecules absorb the heat in your skin.
D)Sweat has a large amount of kinetic energy and that makes it easy to evaporate.
E)none of the above
A)The water molecules leaving the skin as vapor absorb the heat from your skin.
B)The sweat glands require energy to produce water.
C)The water molecules absorb the heat in your skin.
D)Sweat has a large amount of kinetic energy and that makes it easy to evaporate.
E)none of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
68
It easier to cool off on a dry day vs. a humid day because the rate of evaporation is ________.
A)greater on a dry day and more heat is lost
B)slower on a dry day and more heat is lost
C)slower on a dry day and less heat is lost
D)greater on a dry day and less heat is lost
E)none of the above
A)greater on a dry day and more heat is lost
B)slower on a dry day and more heat is lost
C)slower on a dry day and less heat is lost
D)greater on a dry day and less heat is lost
E)none of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
69
From the diagram above describing the phase of water relative to temperature and pressure, is it possible for ice to transform to water vapor (sublime)without ever becoming liquid? At which point on the diagram does this occur?
A)No, water cannot sublime (become vapor from solid)without first becoming liquid.
B)Yes, water can be made to sublime. This occurs at point D on the diagram.
C)Yes, but only at the exact conditions of point B on the diagram.
D)Yes, but the diagram does not demonstrate the action of water as "dry ice."
A)No, water cannot sublime (become vapor from solid)without first becoming liquid.
B)Yes, water can be made to sublime. This occurs at point D on the diagram.
C)Yes, but only at the exact conditions of point B on the diagram.
D)Yes, but the diagram does not demonstrate the action of water as "dry ice."

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
70
How are pressure and the boiling point of a compound related?
A)As the pressure decreases the boiling point decreases.
B)As the boiling point increases the pressure decreases.
C)As the pressure decreases the boiling point increases.
D)As the boiling point decreases the pressure increases.
E)none of the above
A)As the pressure decreases the boiling point decreases.
B)As the boiling point increases the pressure decreases.
C)As the pressure decreases the boiling point increases.
D)As the boiling point decreases the pressure increases.
E)none of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
71
Does soap increase or decrease water's surface tension?
A)increases
B)decreases
C)Soap does not affect water's surface tension.
D)Both; adding a small amount of soap will first decrease water's surface tension but increasing the amount of soap will reverse the effect.
A)increases
B)decreases
C)Soap does not affect water's surface tension.
D)Both; adding a small amount of soap will first decrease water's surface tension but increasing the amount of soap will reverse the effect.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
72
Boiling is when the ________.
A)vapor pressure of a liquid equals the atmospheric pressure
B)rate of evaporation equals the rate of condensation
C)material no longer undergoes condensation
D)vapor pressure decreases to zero
E)none of the above
A)vapor pressure of a liquid equals the atmospheric pressure
B)rate of evaporation equals the rate of condensation
C)material no longer undergoes condensation
D)vapor pressure decreases to zero
E)none of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
73
From the diagram above describing the phase of xenon relative to temperature and pressure what is the phase of xenon at 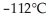 and
and 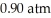 ?
?
A)solid
B)liquid
C)gas
D)melting solid
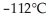 and
and 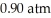 ?
?A)solid
B)liquid
C)gas
D)melting solid

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
74
What happens to the temperature of something while it is boiling?
A)temperature stays the same
B)temperature drops
C)temperature increases
D)Boiling is a cooling process, so temperature should decrease.
E)Boiling requires energy, so the temperature should go up.
A)temperature stays the same
B)temperature drops
C)temperature increases
D)Boiling is a cooling process, so temperature should decrease.
E)Boiling requires energy, so the temperature should go up.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
75
Which statement best describes what is happening at the surface of liquid water?
A)At the surface there is a constant exchange of liquid and gaseous water.
B)Fast moving water molecules leave the surface and become gas molecules.
C)Slow moving gas molecules are captured by the liquid water.
D)The gas molecules exchange with the liquid molecules.
E)all of the above
A)At the surface there is a constant exchange of liquid and gaseous water.
B)Fast moving water molecules leave the surface and become gas molecules.
C)Slow moving gas molecules are captured by the liquid water.
D)The gas molecules exchange with the liquid molecules.
E)all of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
76
Condensation is ________.
A)always a cooling process
B)sometimes a cooling process
C)always a warming process
D)sometimes a warming process
E)Two of the above are correct.
A)always a cooling process
B)sometimes a cooling process
C)always a warming process
D)sometimes a warming process
E)Two of the above are correct.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
77
What is the boiling temperature of a single water molecule? Does this question make sense?
A)Boiling involves the separation of many molecules (plural). With only one molecule, the concept of boiling is meaningless.
B)Yes, this question does make sense because temperature measures the average kinetic energy of a molecule, which is 100°C for water.
C)100°C indicates when the covalent bonds of the water molecule has been broken to give rise to hydrogen and oxygen atoms which are released into atmosphere.
D)No, this question does not make sense because you need at least two molecules to get the average kinetic energy.
A)Boiling involves the separation of many molecules (plural). With only one molecule, the concept of boiling is meaningless.
B)Yes, this question does make sense because temperature measures the average kinetic energy of a molecule, which is 100°C for water.
C)100°C indicates when the covalent bonds of the water molecule has been broken to give rise to hydrogen and oxygen atoms which are released into atmosphere.
D)No, this question does not make sense because you need at least two molecules to get the average kinetic energy.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
78
From the diagram above describing the phase of water relative to temperature and pressure, at which point is water boiling?
A)A
B)B
C)C
D)E
A)A
B)B
C)C
D)E

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
79
Evaporation is ________.
A)always a cooling process
B)sometimes a cooling process
C)always a warming process
D)sometimes a warming process
E)Two of the above are correct.
A)always a cooling process
B)sometimes a cooling process
C)always a warming process
D)sometimes a warming process
E)Two of the above are correct.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
80
A gold ring cannot sit on the surface of water, but a thin gold wire loop of the same diameter can. Why?
A)The gold ring applies a much greater pressure against the surface of the water so that it is able to push through the surface tension.
B)The surface area of contact is the same, but the weight of the gold ring is much greater.
C)The thin gold wire is far less dense than the gold ring, which allows it to float.
D)Both A and B are true.
A)The gold ring applies a much greater pressure against the surface of the water so that it is able to push through the surface tension.
B)The surface area of contact is the same, but the weight of the gold ring is much greater.
C)The thin gold wire is far less dense than the gold ring, which allows it to float.
D)Both A and B are true.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck


