Deck 4: Aqueous Reactions and Solution Stoichiometry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/177
Play
Full screen (f)
Deck 4: Aqueous Reactions and Solution Stoichiometry
1
With which of the following will the ammonium ion form an insoluble salt?
A)chloride
B)sulfate
C)carbonate
D)sulfate and carbonate
E)none of the above
A)chloride
B)sulfate
C)carbonate
D)sulfate and carbonate
E)none of the above
none of the above
2
When aqueous solutions of __________ are mixed, a precipitate forms.
A)NiBr2 and AgNO3
B)NaI and KBr
C)K2SO4 and CrCl3
D)KOH and Ba(NO3)2
E)Li2CO3 and CsI
A)NiBr2 and AgNO3
B)NaI and KBr
C)K2SO4 and CrCl3
D)KOH and Ba(NO3)2
E)Li2CO3 and CsI
NiBr2 and AgNO3
3
Which of the following is insoluble in water at 25 °C?
A)Mg3(PO4)2
B)Na2S
C)(NH4)2CO3
D)Ca(OH)2
E)Ba(C2H3O2)2
A)Mg3(PO4)2
B)Na2S
C)(NH4)2CO3
D)Ca(OH)2
E)Ba(C2H3O2)2
Mg3(PO4)2
4
Which one of the following compounds is insoluble in water?
A)K2SO4
B)Ca(C2H3O2)2
C)MgC2O4
D)ZnCl2
E)Mn(NO3)2
A)K2SO4
B)Ca(C2H3O2)2
C)MgC2O4
D)ZnCl2
E)Mn(NO3)2

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
5
Of the species below, only __________ is not an electrolyte.
A)HCl
B)Rb2SO4
C)Ar
D)KOH
E)NaCl
A)HCl
B)Rb2SO4
C)Ar
D)KOH
E)NaCl

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
6
Which combination will produce a precipitate?
A)NH4OH (aq)and HCl (aq)
B)AgNO3 (aq)and Ca(C2H3O2)2 (aq)
C)NaOH (aq)and HCl (aq)
D)NaCl (aq)and H C2H3O2 (aq)
E)NaOH (aq)and Fe(NO3)2 (aq)
A)NH4OH (aq)and HCl (aq)
B)AgNO3 (aq)and Ca(C2H3O2)2 (aq)
C)NaOH (aq)and HCl (aq)
D)NaCl (aq)and H C2H3O2 (aq)
E)NaOH (aq)and Fe(NO3)2 (aq)

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
7
The reaction between strontium hydroxide and chloric acid produces __________.
A)a molecular compound and a weak electrolyte
B)two weak electrolytes
C)two strong electrolytes
D)a molecular compound and a strong electrolyte
E)two molecular compounds
A)a molecular compound and a weak electrolyte
B)two weak electrolytes
C)two strong electrolytes
D)a molecular compound and a strong electrolyte
E)two molecular compounds

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
8
The net ionic equation for formation of an aqueous solution of NiI2 accompanied by evolution of CO2 gas via mixing solid NiCO3 and aqueous hydriodic acid is __________.
A)2NiCO3 (s)+ HI (aq)→ 2 H2O (l)+ CO2 (g)+ 2Ni2+ (aq)
B)NiCO3 (s)+ I- (aq)→ 2 H2O (l)+ CO2 (g)+ Ni2+ (aq)+ HI (aq)
C)NiCO3 (s)+ 2H+ (aq)→ H2O (l)+ CO2 (g)+ Ni2+ (aq)
D)NiCO3 (s)+ 2HI (aq)→ 2 H2O (l)+ CO2 (g)+ NiI2 (aq)
E)NiCO3 (s)+ 2HI (aq)→ H2O (l)+ CO2 (g)+ Ni2+ (aq)+ 2 I- (aq)
A)2NiCO3 (s)+ HI (aq)→ 2 H2O (l)+ CO2 (g)+ 2Ni2+ (aq)
B)NiCO3 (s)+ I- (aq)→ 2 H2O (l)+ CO2 (g)+ Ni2+ (aq)+ HI (aq)
C)NiCO3 (s)+ 2H+ (aq)→ H2O (l)+ CO2 (g)+ Ni2+ (aq)
D)NiCO3 (s)+ 2HI (aq)→ 2 H2O (l)+ CO2 (g)+ NiI2 (aq)
E)NiCO3 (s)+ 2HI (aq)→ H2O (l)+ CO2 (g)+ Ni2+ (aq)+ 2 I- (aq)

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
9
The net ionic equation for the reaction between aqueous sulfuric acid and aqueous sodium hydroxide is __________.
A)H+ (aq)+ HSO4- (aq)+ 2OH- (aq)→ 2 H2O (l)+ SO42- (aq)
B)H+ (aq)+ HSO4- (aq)+ 2 Na+ (aq)+ 2OH- (aq)→ 2 H2O (l)+ 2Na+ (aq)+ SO42- (aq)
C)SO42- (aq)+ 2Na+ (aq)→ 2Na+ (aq)+ SO42- (aq)
D)H+ (aq)+ OH- (aq)→ H2O( l)
E)2H+ (aq)+ SO42- (aq)+ 2Na+ (aq)+ 2OH- (aq)→ 2 H2O (l)+ 2Na+ (aq)+ SO42- (aq)
A)H+ (aq)+ HSO4- (aq)+ 2OH- (aq)→ 2 H2O (l)+ SO42- (aq)
B)H+ (aq)+ HSO4- (aq)+ 2 Na+ (aq)+ 2OH- (aq)→ 2 H2O (l)+ 2Na+ (aq)+ SO42- (aq)
C)SO42- (aq)+ 2Na+ (aq)→ 2Na+ (aq)+ SO42- (aq)
D)H+ (aq)+ OH- (aq)→ H2O( l)
E)2H+ (aq)+ SO42- (aq)+ 2Na+ (aq)+ 2OH- (aq)→ 2 H2O (l)+ 2Na+ (aq)+ SO42- (aq)

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
10
Which combination will produce a precipitate?
A)Pb(NO3)2 (aq)and HCl (aq)
B)Cu(NO3)2 (aq)and KC2H3O2 (aq)
C)KOH (aq)and HNO3 (aq)
D)AgC2H3O2 (aq)and HC2H3O2 (aq)
E)NaOH (aq)and Sr(NO3)2 (aq)
A)Pb(NO3)2 (aq)and HCl (aq)
B)Cu(NO3)2 (aq)and KC2H3O2 (aq)
C)KOH (aq)and HNO3 (aq)
D)AgC2H3O2 (aq)and HC2H3O2 (aq)
E)NaOH (aq)and Sr(NO3)2 (aq)

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
11
The net ionic equation for formation of an aqueous solution of Al(NO3)3 via mixing solid Al(OH)3 and aqueous nitric acid is __________.
A)Al(OH)3 (s)+ 3HNO3 (aq)→ 3 H2O (l)+ Al(NO3)3 (aq)
B)Al(OH)3 (s)+ 3NO3- (aq)→ 3OH- (aq)+ Al(NO3)3 (aq)
C)Al(OH)3 (s)+ 3NO3- (aq)→ 3OH- (aq)+ Al(NO3)3 (s)
D)Al(OH)3 (s)+ 3H+ (aq)→ 3 H2O (l)+ Al3+ (aq)
E)Al(OH)3 (s)+ 3HNO3 (aq)→ 3 H2O (l)+ Al3+ (aq)+ NO3- (aq)
A)Al(OH)3 (s)+ 3HNO3 (aq)→ 3 H2O (l)+ Al(NO3)3 (aq)
B)Al(OH)3 (s)+ 3NO3- (aq)→ 3OH- (aq)+ Al(NO3)3 (aq)
C)Al(OH)3 (s)+ 3NO3- (aq)→ 3OH- (aq)+ Al(NO3)3 (s)
D)Al(OH)3 (s)+ 3H+ (aq)→ 3 H2O (l)+ Al3+ (aq)
E)Al(OH)3 (s)+ 3HNO3 (aq)→ 3 H2O (l)+ Al3+ (aq)+ NO3- (aq)

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
12
Aqueous potassium chloride will react with which one of the following in an exchange (metathesis)reaction?
A)calcium nitrate
B)sodium bromide
C)lead nitrate
D)barium nitrate
E)sodium chloride
A)calcium nitrate
B)sodium bromide
C)lead nitrate
D)barium nitrate
E)sodium chloride

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
13
With which of the following will the potassium ion form an insoluble salt?
A)chloride
B)sulfate
C)carbonate
D)sulfate and carbonate
E)none of the above
A)chloride
B)sulfate
C)carbonate
D)sulfate and carbonate
E)none of the above

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
14
Which combination will produce a precipitate?
A)NaC2H3O2 (aq)and HCl (aq)
B)NaOH (aq)and HCl (aq)
C)AgNO3 (aq)and Ca(C2H3O2)2 (aq)
D)KOH (aq)and Mg(NO3)2 (aq)
A)NaC2H3O2 (aq)and HCl (aq)
B)NaOH (aq)and HCl (aq)
C)AgNO3 (aq)and Ca(C2H3O2)2 (aq)
D)KOH (aq)and Mg(NO3)2 (aq)

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
15
The net ionic equation for the reaction between aqueous nitric acid and aqueous sodium hydroxide is __________.
A)H+ (aq)+ HNO3 (aq)+ 2OH- (aq)→ 2 H2O (l)+ NO3- (aq)
B)HNO3 (aq)+ NaOH (aq)→ NaNO3 (aq)+ H2O (l)
C)H+ (aq)+ OH- (aq)→ H2O (l)
D)HNO3 (aq)+ OH- (aq)→ NO3- (aq)+ H2O (l)
E)H+ (aq)+ Na+ (aq)+OH- (aq)→ H2O (l)+ Na+ (aq)
A)H+ (aq)+ HNO3 (aq)+ 2OH- (aq)→ 2 H2O (l)+ NO3- (aq)
B)HNO3 (aq)+ NaOH (aq)→ NaNO3 (aq)+ H2O (l)
C)H+ (aq)+ OH- (aq)→ H2O (l)
D)HNO3 (aq)+ OH- (aq)→ NO3- (aq)+ H2O (l)
E)H+ (aq)+ Na+ (aq)+OH- (aq)→ H2O (l)+ Na+ (aq)

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
16
Aqueous solutions of a compound did not form precipitates with Cl-, Br-, I-, SO42- , CO32-, PO43-, OH-, or S2-. This highly water-soluble compound produced the foul-smelling gas H2S when the solution was acidified. This compound is __________.
A)Pb(NO3)2
B)(NH4)2S
C)KBr
D)Li2CO3
E)AgNO3
A)Pb(NO3)2
B)(NH4)2S
C)KBr
D)Li2CO3
E)AgNO3

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is soluble in water at 25 °C?
A)Fe3 (PO4)2
B)Fe(OH)2
C)Fe(NO3)2
D)FeCO3
E)FeS
A)Fe3 (PO4)2
B)Fe(OH)2
C)Fe(NO3)2
D)FeCO3
E)FeS

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
18
Which one of the following is a diprotic acid?
A)nitric acid
B)chloric acid
C)phosphoric acid
D)hydrofluoric acid
E)sulfuric acid
A)nitric acid
B)chloric acid
C)phosphoric acid
D)hydrofluoric acid
E)sulfuric acid

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
19
The balanced molecular equation for complete neutralization of H2SO4 by KOH in aqueous solution is __________.
A)2H+ (aq)+ 2OH- (aq)→ 2 H2O (l)
B)2H+ (aq)+ 2KOH (aq)→ 2 H2O (l)+ 2K+ (aq)
C)H2SO4 (aq)+ 2OH- (aq)→ 2 H2O (l)+ SO42- (aq)
D)H2SO4 (aq)+ 2KOH (aq)→ 2 H2O (l)+ K2SO4 (s)
E)H2SO4 (aq)+ 2KOH (aq)→ 2 H2O (l)+ K2SO4 (aq)
A)2H+ (aq)+ 2OH- (aq)→ 2 H2O (l)
B)2H+ (aq)+ 2KOH (aq)→ 2 H2O (l)+ 2K+ (aq)
C)H2SO4 (aq)+ 2OH- (aq)→ 2 H2O (l)+ SO42- (aq)
D)H2SO4 (aq)+ 2KOH (aq)→ 2 H2O (l)+ K2SO4 (s)
E)H2SO4 (aq)+ 2KOH (aq)→ 2 H2O (l)+ K2SO4 (aq)

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
20
Which one of the following compounds is insoluble in water?
A)Na2CO3
B)K2SO4
C)Fe(NO3)3
D)ZnS
E)AgNO3
A)Na2CO3
B)K2SO4
C)Fe(NO3)3
D)ZnS
E)AgNO3

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
21
In which species does sulfur have the highest oxidation number?
A)S8 (elemental form of sulfur)
B)H2S
C)SO2
D)H2SO3
E)K2SO4
A)S8 (elemental form of sulfur)
B)H2S
C)SO2
D)H2SO3
E)K2SO4

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
22
In which reaction does the oxidation number of hydrogen change?
A)HCl (aq)+ NaOH (aq)→ NaCl (aq)+ H2O (l)
B)2 Na (s)+ 2 H2O (l)→ 2 NaOH (aq)+ H2 (g)
C)CaO (s)+ H2O (l)→ Ca(OH)2 (s)
D)2 HClO4 (aq)+ CaCO3 (s)→ Ca(ClO4)2 (aq)+ H2O (l)+ CO2 (g)
E)SO2 (g)+ H2O (l)→ H2SO3 (aq)
A)HCl (aq)+ NaOH (aq)→ NaCl (aq)+ H2O (l)
B)2 Na (s)+ 2 H2O (l)→ 2 NaOH (aq)+ H2 (g)
C)CaO (s)+ H2O (l)→ Ca(OH)2 (s)
D)2 HClO4 (aq)+ CaCO3 (s)→ Ca(ClO4)2 (aq)+ H2O (l)+ CO2 (g)
E)SO2 (g)+ H2O (l)→ H2SO3 (aq)

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
23
A compound was found to be soluble in water. It was also found that addition of acid to an aqueous solution of this compound resulted in the formation of carbon dioxide. Which one of the following cations would form a precipitate when added to an aqueous solution of this compound?
A)NH4+
B)K+
C)Cr3+
D)Rb+
E)Na+
A)NH4+
B)K+
C)Cr3+
D)Rb+
E)Na+

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
24
Which of these metals will be oxidized by the ions of cobalt?
A)nickel
B)tin
C)iron
D)copper
E)silver
A)nickel
B)tin
C)iron
D)copper
E)silver

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
25
In which species does nitrogen have the highest oxidation number?
A)N2
B)NH3
C)HNO2
D)NO2-
E)NaNO3
A)N2
B)NH3
C)HNO2
D)NO2-
E)NaNO3

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
26
In which reaction does the oxidation number of oxygen increase?
A)Ba(NO3)2 (aq)+ K2SO4 (aq)→ BaSO4 (s)+ 2 KNO3 (aq)
B)HCl (aq)+ NaOH (aq)→ NaCl (aq)+ H2O (l)
C)MgO (s)+ H2O (l)→ Mg(OH)2 (s)
D)2 SO2 (g)+ O2 (g)→ 2 SO3 (g)
E)2 H2O (l)→ 2 H2 (g)+ O2 (g)
A)Ba(NO3)2 (aq)+ K2SO4 (aq)→ BaSO4 (s)+ 2 KNO3 (aq)
B)HCl (aq)+ NaOH (aq)→ NaCl (aq)+ H2O (l)
C)MgO (s)+ H2O (l)→ Mg(OH)2 (s)
D)2 SO2 (g)+ O2 (g)→ 2 SO3 (g)
E)2 H2O (l)→ 2 H2 (g)+ O2 (g)

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
27
Based on the activity series, which one of the reactions below will occur?
A)Zn (s)+ MnI2 (aq)→ ZnI2 (aq)+ Mn (s)
B)SnCl2 (aq)+ Cu (s)→ Sn (s)+ CuCl2 (aq)
C)2AgNO3 (aq)+ Pb (s)→ 2Ag (s)+ Pb(NO3)2 (aq)
D)3Hg (l)+ 2Cr(NO3)3 (aq)→ 3Hg(NO3)2 + 2Cr (s)
E)3FeBr2 (aq)+ 2Au (s)→ 3Fe (s)+ 2AuBr3 (aq)
A)Zn (s)+ MnI2 (aq)→ ZnI2 (aq)+ Mn (s)
B)SnCl2 (aq)+ Cu (s)→ Sn (s)+ CuCl2 (aq)
C)2AgNO3 (aq)+ Pb (s)→ 2Ag (s)+ Pb(NO3)2 (aq)
D)3Hg (l)+ 2Cr(NO3)3 (aq)→ 3Hg(NO3)2 + 2Cr (s)
E)3FeBr2 (aq)+ 2Au (s)→ 3Fe (s)+ 2AuBr3 (aq)

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
28
Which compound has the atom with the highest oxidation number?
A)CaS
B)Na3N
C)MgSO3
D)Al(NO2)3
E)NH4Cl
A)CaS
B)Na3N
C)MgSO3
D)Al(NO2)3
E)NH4Cl

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
29
One method for removal of metal ions from a solution is to convert the metal to its elemental form so it can be filtered out as a solid. Which metal can be used to remove aluminum ions from solution?
A)zinc
B)cobalt
C)lead
D)copper
E)none of these
A)zinc
B)cobalt
C)lead
D)copper
E)none of these

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
30
The balanced reaction between aqueous potassium hydroxide and aqueous acetic acid is __________.
A)KOH (aq)+ HC2H3O2 (aq)→ OH- (l)+ H C2H3O2+ (aq)+ K (s)
B)KOH (aq)+ HC2H3O2 (aq)→ H2O (l)+ KC2H3O2 ( aq)
C)KOH (aq)+ HC2H3O2 (aq)→ H2C2H3O3 (aq)+ K (s)
D)KOH (aq)+ HC2H3O2 (aq)→ KC2H3O3 (aq)+ H2 (g)
E)KOH (aq)+ HC2H3O2 (aq)→ H2KC2H3O (aq)+ O2 (g)
A)KOH (aq)+ HC2H3O2 (aq)→ OH- (l)+ H C2H3O2+ (aq)+ K (s)
B)KOH (aq)+ HC2H3O2 (aq)→ H2O (l)+ KC2H3O2 ( aq)
C)KOH (aq)+ HC2H3O2 (aq)→ H2C2H3O3 (aq)+ K (s)
D)KOH (aq)+ HC2H3O2 (aq)→ KC2H3O3 (aq)+ H2 (g)
E)KOH (aq)+ HC2H3O2 (aq)→ H2KC2H3O (aq)+ O2 (g)

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
31
Which one of the following solutions will have the greatest concentration of hydroxide ions?
A)0.300 M rubidium hydroxide
B)0.100 M magnesium hydroxide
C)0.100 M ammonia
D)0.100 M beryllium hydroxide
E)0.100 M hydrochloric acid
A)0.300 M rubidium hydroxide
B)0.100 M magnesium hydroxide
C)0.100 M ammonia
D)0.100 M beryllium hydroxide
E)0.100 M hydrochloric acid

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
32
Which one of the following is a weak acid?
A)HNO3
B)HCl
C)HI
D)HF
E)HClO4
A)HNO3
B)HCl
C)HI
D)HF
E)HClO4

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
33
Of the choices below, which would be the best for the lining of a tank intended for use in storage of hydrochloric acid?
A)copper
B)zinc
C)nickel
D)iron
E)tin
A)copper
B)zinc
C)nickel
D)iron
E)tin

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following are weak acids?
A)HF, HBr
B)HI, HNO3, HBr
C)HI, HF
D)HF
E)none of the above
A)HF, HBr
B)HI, HNO3, HBr
C)HI, HF
D)HF
E)none of the above

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
35
Which one of the following is a triprotic acid?
A)nitric acid
B)chloric acid
C)phosphoric acid
D)hydrofluoric acid
E)sulfuric acid
A)nitric acid
B)chloric acid
C)phosphoric acid
D)hydrofluoric acid
E)sulfuric acid

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
36
Of the reactions below, only __________ is not spontaneous.
A)Mg (s)+ 2HCl (aq)→ MgCl2 (aq)+ H2 (g)
B)2Ag (s)+ 2HNO3 (aq)→ 2AgNO3 (aq)+ H2 (g)
C)2Ni (s)+ H2SO4 (aq)→ Ni2SO4 (aq)+ H2 (g)
D)2Al (s)+ 6HBr (aq)→ 2AlBr3 (aq)+ 3 H2 (g)
E)Zn (s)+ 2HI (aq)→ ZnI2 (aq)+ H2 (g)
A)Mg (s)+ 2HCl (aq)→ MgCl2 (aq)+ H2 (g)
B)2Ag (s)+ 2HNO3 (aq)→ 2AgNO3 (aq)+ H2 (g)
C)2Ni (s)+ H2SO4 (aq)→ Ni2SO4 (aq)+ H2 (g)
D)2Al (s)+ 6HBr (aq)→ 2AlBr3 (aq)+ 3 H2 (g)
E)Zn (s)+ 2HI (aq)→ ZnI2 (aq)+ H2 (g)

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
37
The balanced reaction between aqueous nitric acid and aqueous strontium hydroxide is __________.
A)HNO3 (aq)+ Sr(OH)2 (aq)→ Sr(NO3)2 (aq)+ H2 (g)
B)HNO3 (aq)+ Sr(OH)2 (aq)→ H2O (l)+ Sr(NO3)2 (aq)
C)HNO3 (aq)+ SrOH (aq)→ H2O (l)+ SrNO3 (aq)
D)2HNO3 (aq)+ Sr(OH)2 (aq)→ 2 H2O (l)+ Sr(NO3)2 (aq)
E)2HNO3 (aq)+ Sr(OH)2 (aq)→ Sr(NO3)2 (aq)+ 2 H2 (g)
A)HNO3 (aq)+ Sr(OH)2 (aq)→ Sr(NO3)2 (aq)+ H2 (g)
B)HNO3 (aq)+ Sr(OH)2 (aq)→ H2O (l)+ Sr(NO3)2 (aq)
C)HNO3 (aq)+ SrOH (aq)→ H2O (l)+ SrNO3 (aq)
D)2HNO3 (aq)+ Sr(OH)2 (aq)→ 2 H2O (l)+ Sr(NO3)2 (aq)
E)2HNO3 (aq)+ Sr(OH)2 (aq)→ Sr(NO3)2 (aq)+ 2 H2 (g)

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
38
Which of these metals will be oxidized by the ions of aluminum?
A)magnesium
B)zinc
C)chromium
D)iron
E)nickel
A)magnesium
B)zinc
C)chromium
D)iron
E)nickel

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
39
Which hydroxides are weak bases?
A)KOH, Ba(OH)2
B)Sr(OH)2, KOH, NaOH, Ba(OH)2
C)KOH, NaOH
D)KOH, NaOH, Ba(OH)2
E)None of these is a weak base.
A)KOH, Ba(OH)2
B)Sr(OH)2, KOH, NaOH, Ba(OH)2
C)KOH, NaOH
D)KOH, NaOH, Ba(OH)2
E)None of these is a weak base.

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
40
Based on the activity series, which one of the reactions below will occur?
A)Fe (s)+ ZnCl2 (aq)→ FeCl2 (aq)+ Zn (s)
B)Mn (s)+ NiCl2 (aq)→ MnCl2 (aq)+ Ni (s)
C)Pb (s)+ NiI2 (aq)→ PbI2 (aq)+ Ni (s)
D)SnBr2 (aq)+ Cu (s)→ CuBr2 (aq)+ Sn (s)
E)None of the reactions will occur.
A)Fe (s)+ ZnCl2 (aq)→ FeCl2 (aq)+ Zn (s)
B)Mn (s)+ NiCl2 (aq)→ MnCl2 (aq)+ Ni (s)
C)Pb (s)+ NiI2 (aq)→ PbI2 (aq)+ Ni (s)
D)SnBr2 (aq)+ Cu (s)→ CuBr2 (aq)+ Sn (s)
E)None of the reactions will occur.

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
41
A tenfold dilution of a sample solution can be obtained by taking __________.
A)1 part sample and 9 parts solvent
B)1 part sample and 10 parts solvent
C)9 parts sample and 1 part solvent
D)10 parts sample and 1 part solvent
E)99 parts sample and 1 part solvent
A)1 part sample and 9 parts solvent
B)1 part sample and 10 parts solvent
C)9 parts sample and 1 part solvent
D)10 parts sample and 1 part solvent
E)99 parts sample and 1 part solvent

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
42
You are given two clear solutions of the same unknown monoprotic acid, but with different concentrations. Which statement is true?
A)There is no chemical method designed to tell the two solutions apart.
B)It would take more base solution (per milliliter of the unknown solution)to neutralize the more concentrated solution.
C)A smaller volume of the less concentrated solution contains the same number of moles of the acid compared to the more concentrated solution.
D)If the same volume of each sample was taken, then more base solution would be required to neutralize the one with lower concentration.
E)The product of concentration and volume of the less concentrated solution equals the product of concentration and volume of the more concentrated solution.
A)There is no chemical method designed to tell the two solutions apart.
B)It would take more base solution (per milliliter of the unknown solution)to neutralize the more concentrated solution.
C)A smaller volume of the less concentrated solution contains the same number of moles of the acid compared to the more concentrated solution.
D)If the same volume of each sample was taken, then more base solution would be required to neutralize the one with lower concentration.
E)The product of concentration and volume of the less concentrated solution equals the product of concentration and volume of the more concentrated solution.

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
43
What are the respective concentrations (M)of Cu+2 and Cl- afforded by dissolving 0.200 mol CuCl2 in water and diluting to 345 mL?
A)0.200 and 0.200
B)0.580 and 1.16
C)0.200 and 0.400
D)1.16 and 2.32
E)0.580 and 0.290
A)0.200 and 0.200
B)0.580 and 1.16
C)0.200 and 0.400
D)1.16 and 2.32
E)0.580 and 0.290

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following is an oxidation-reduction reaction?
A)Cu (s)+ 2AgNO3 (aq)→ 2Ag (s)+ Cu(NO3)2 (aq)
B)HCl (aq)+ NaOH (aq)→ H2O (l)+ NaCl (aq)
C)AgNO3 (aq)+ HCl (aq)→ AgCl (s)+ HNO3 (aq)
D)Ba(C2H3O2)2 (aq)+ Na2SO4 (aq)→ BaSO4 (s)+ 2NaC2H3O2 (aq)
E)H2CO3 (aq)+ Ca(NO3)2 (aq)→ 2HNO3 (aq)+ CaCO3 (s)
A)Cu (s)+ 2AgNO3 (aq)→ 2Ag (s)+ Cu(NO3)2 (aq)
B)HCl (aq)+ NaOH (aq)→ H2O (l)+ NaCl (aq)
C)AgNO3 (aq)+ HCl (aq)→ AgCl (s)+ HNO3 (aq)
D)Ba(C2H3O2)2 (aq)+ Na2SO4 (aq)→ BaSO4 (s)+ 2NaC2H3O2 (aq)
E)H2CO3 (aq)+ Ca(NO3)2 (aq)→ 2HNO3 (aq)+ CaCO3 (s)

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
45
Which one of the following is not true concerning 2.00 L of 0.100 M solution of Ca3(PO4)2?
A)This solution contains 0.200 mol of Ca3(PO4)2.
B)This solution contains 0.800 mol of oxygen atoms.
C)1.00 L of this solution is required to furnish 0.300 mol of Ca2+ ions.
D)There are 6.02 × 1022 phosphorus atoms in 500.0 mL of this solution.
E)This solution contains 6.67 × 10-2 mol of Ca2+.
A)This solution contains 0.200 mol of Ca3(PO4)2.
B)This solution contains 0.800 mol of oxygen atoms.
C)1.00 L of this solution is required to furnish 0.300 mol of Ca2+ ions.
D)There are 6.02 × 1022 phosphorus atoms in 500.0 mL of this solution.
E)This solution contains 6.67 × 10-2 mol of Ca2+.

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
46
Which solution has the same number of moles of KCl as 75.00 mL of 0.250M solution of KCl?
A)20.0 mL of 0.200M solution of KCl
B)25.0 mL of 0.175M solution of KCl
C)129 mL of 0.145M solution of KCl
D)50.0 mL of 0.125M solution of KCl
E)100 mL of 0.0500M solution of KCl
A)20.0 mL of 0.200M solution of KCl
B)25.0 mL of 0.175M solution of KCl
C)129 mL of 0.145M solution of KCl
D)50.0 mL of 0.125M solution of KCl
E)100 mL of 0.0500M solution of KCl

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
47
Mixing 10.00 mL of an aqueous solution with 10.00 mL of water represents a __________.
A)crystallization
B)neutralization
C)twofold dilution
D)tenfold dilution
E)titration
A)crystallization
B)neutralization
C)twofold dilution
D)tenfold dilution
E)titration

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
48
A 0.100 M solution of __________ will contain the highest concentration of potassium ions.
A)potassium phosphate
B)potassium hydrogen carbonate
C)potassium hypochlorite
D)potassium iodide
E)potassium oxide
A)potassium phosphate
B)potassium hydrogen carbonate
C)potassium hypochlorite
D)potassium iodide
E)potassium oxide

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
49
Oxidation is the __________ and reduction is the __________.
A)gain of oxygen, loss of electrons
B)loss of oxygen, gain of electrons
C)loss of electrons, gain of electrons
D)gain of oxygen, loss of mass
E)gain of electrons, loss of electrons
A)gain of oxygen, loss of electrons
B)loss of oxygen, gain of electrons
C)loss of electrons, gain of electrons
D)gain of oxygen, loss of mass
E)gain of electrons, loss of electrons

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
50
What are the respective concentrations (M)of Fe3+ and I- afforded by dissolving 0.200 mol FeI3 in water and diluting to 725 mL?
A)0.276 and 0.828
B)0.828 and 0.276
C)0.276 and 0.276
D)0.145 and 0.435
E)0.145 and 0.0483
A)0.276 and 0.828
B)0.828 and 0.276
C)0.276 and 0.276
D)0.145 and 0.435
E)0.145 and 0.0483

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
51
Oxidation cannot occur without __________.
A)acid
B)oxygen
C)water
D)air
E)reduction
A)acid
B)oxygen
C)water
D)air
E)reduction

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
52
The net ionic equation for the dissolution of zinc metal in aqueous hydrobromic acid is __________.
A)Zn (s)+ 2Br- (aq)→ ZnBr2 (aq)
B)Zn (s)+ 2HBr (aq)→ ZnBr2 (aq)+ 2H+ (aq)
C)Zn (s)+ 2HBr (aq)→ ZnBr2 (s)+ 2H+ (aq)
D)Zn (s)+ 2H+ (aq)→ Zn2+ (aq)+ H2 (g)
E)2Zn (s)+ H+ (aq)→ 2Zn2+ (aq)+ H2 (g)
A)Zn (s)+ 2Br- (aq)→ ZnBr2 (aq)
B)Zn (s)+ 2HBr (aq)→ ZnBr2 (aq)+ 2H+ (aq)
C)Zn (s)+ 2HBr (aq)→ ZnBr2 (s)+ 2H+ (aq)
D)Zn (s)+ 2H+ (aq)→ Zn2+ (aq)+ H2 (g)
E)2Zn (s)+ H+ (aq)→ 2Zn2+ (aq)+ H2 (g)

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
53
Zinc is more active than cobalt and iron but less active than aluminum. Cobalt is more active than nickel but less active than iron. Which of the following correctly lists the elements in order of increasing activity?
A)Co < Ni < Fe < Zn < Al
B)Ni < Fe < Co < Zn < Al
C)Ni < Co < Fe < Zn < Al
D)Fe < Ni < Co < Al < Zn
E)Zn < Al < Co < Ni < Fe
A)Co < Ni < Fe < Zn < Al
B)Ni < Fe < Co < Zn < Al
C)Ni < Co < Fe < Zn < Al
D)Fe < Ni < Co < Al < Zn
E)Zn < Al < Co < Ni < Fe

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
54
Oxidation and __________ mean essentially the same thing.
A)activity
B)reduction
C)metathesis
D)decomposition
E)corrosion
A)activity
B)reduction
C)metathesis
D)decomposition
E)corrosion

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
55
What are the respective concentrations (M)of Mg+2 and C2H3O2- afforded by dissolving 0.600 mol Mg(C2H3O2)2 in water and diluting to 135 mL?
A)0.444 and 0.889
B)0.0444 and 0.0889
C)0..889 and 0.444
D)0.444 and 0.444
E)4.44 and 8.89
A)0.444 and 0.889
B)0.0444 and 0.0889
C)0..889 and 0.444
D)0.444 and 0.444
E)4.44 and 8.89

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following reactions will not occur as written?
A)Zn (s)+ Pb(NO3)2 (aq)→ Pb (s)+ Zn(NO3)2 (aq)
B)Mg (s)+ Ca(OH)2 (aq)→ Ca (s)+ Mg(OH)2 (aq)
C)Sn (s)+ 2AgNO3 (aq)→ 2Ag (s)+ Sn(NO3)2 (aq)
D)Co (s)+ 2AgCl (aq)→ 2Ag (s)+ CoCl2 (aq)
E)Co (s)+ 2HI (aq)→ H2 (g)+ CoI2 (aq)
A)Zn (s)+ Pb(NO3)2 (aq)→ Pb (s)+ Zn(NO3)2 (aq)
B)Mg (s)+ Ca(OH)2 (aq)→ Ca (s)+ Mg(OH)2 (aq)
C)Sn (s)+ 2AgNO3 (aq)→ 2Ag (s)+ Sn(NO3)2 (aq)
D)Co (s)+ 2AgCl (aq)→ 2Ag (s)+ CoCl2 (aq)
E)Co (s)+ 2HI (aq)→ H2 (g)+ CoI2 (aq)

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
57
Which solution has the same number of moles of NaOH as 50.00 mL of 0.100M solution of NaOH?
A)20.00 mL of 0.200M solution of NaOH
B)25.00 mL of 0.175M solution of NaOH
C)30.00 mL of 0.145M solution of NaOH
D)50.00 mL of 0.125M solution of NaOH
E)100.00 mL of 0.0500M solution of NaOH
A)20.00 mL of 0.200M solution of NaOH
B)25.00 mL of 0.175M solution of NaOH
C)30.00 mL of 0.145M solution of NaOH
D)50.00 mL of 0.125M solution of NaOH
E)100.00 mL of 0.0500M solution of NaOH

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
58
A 0.200 M K2SO4 solution is produced by __________.
A)dilution of 250.0 mL of 1.00 M K2SO4 to 1.00 L
B)dissolving 43.6 g of K2SO4 in water and diluting to a total volume of 250.0 mL
C)diluting 20.0 mL of 5.00 M K2SO4 solution to 500.0 mL
D)dissolving 20.2 g of K2SO4 in water and diluting to 250.0 mL, then diluting 25.0 mL of this solution to a total volume of 500.0 mL
E)dilution of 1.00 mL of 250 M K2SO3 to 1.00 L
A)dilution of 250.0 mL of 1.00 M K2SO4 to 1.00 L
B)dissolving 43.6 g of K2SO4 in water and diluting to a total volume of 250.0 mL
C)diluting 20.0 mL of 5.00 M K2SO4 solution to 500.0 mL
D)dissolving 20.2 g of K2SO4 in water and diluting to 250.0 mL, then diluting 25.0 mL of this solution to a total volume of 500.0 mL
E)dilution of 1.00 mL of 250 M K2SO3 to 1.00 L

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
59
Which one of the following is a correct expression for molarity?
A)mol solute/L solvent
B)mol solute/mL solvent
C)mmol solute/mL solution
D)mol solute/kg solvent
E)μmol solute/L solution
A)mol solute/L solvent
B)mol solute/mL solvent
C)mmol solute/mL solution
D)mol solute/kg solvent
E)μmol solute/L solution

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
60
Sodium does not occur in nature as Na (s)because __________.
A)it is easily reduced to Na-
B)it is easily oxidized to Na+
C)it reacts with water with great difficulty
D)it is easily replaced by silver in its ores
E)it undergoes a disproportionation reaction to Na- and Na+
A)it is easily reduced to Na-
B)it is easily oxidized to Na+
C)it reacts with water with great difficulty
D)it is easily replaced by silver in its ores
E)it undergoes a disproportionation reaction to Na- and Na+

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
61
Which one of the following substances is produced during the reaction of an acid with a metal hydroxide?
A)H2
B)H2O
C)CO2
D)NaOH
E)O2
A)H2
B)H2O
C)CO2
D)NaOH
E)O2

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following would require the largest volume of 0.100 M sodium hydroxide solution for neutralization?
A)10.0 mL of 0.0500 M phosphoric acid
B)20.0 mL of 0.0500 M nitric acid
C)5.0 mL of 0.0100 M sulfuric acid
D)15.0 mL of 0.0500 M hydrobromic acid
E)10.0 mL of 0.0500 M perchloric acid
A)10.0 mL of 0.0500 M phosphoric acid
B)20.0 mL of 0.0500 M nitric acid
C)5.0 mL of 0.0100 M sulfuric acid
D)15.0 mL of 0.0500 M hydrobromic acid
E)10.0 mL of 0.0500 M perchloric acid

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
63
What volume (mL)of 0.135 M NaOH is required to neutralize 13.7 mL of 0.129 M HCl?
A)13.1
B)0.24
C)14.3
D)0.076
E)6.55
A)13.1
B)0.24
C)14.3
D)0.076
E)6.55

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
64
What mass (g)of barium iodide is contained in 250 mL of a barium iodide solution that has an iodide ion concentration of 0.193 M?
A)9.44
B)18.9
C)0.024
D)0.048
E)37.7
A)9.44
B)18.9
C)0.024
D)0.048
E)37.7

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
65
What mass (g)of AgBr is formed when 35.5 mL of 0.184 M AgNO3 is treated with an excess of aqueous hydrobromic acid?
A)1.44
B)1.23
C)53.6
D)34.5
E)188
A)1.44
B)1.23
C)53.6
D)34.5
E)188

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
66
Which solution contains the largest number of moles of chloride ions?
A)10.0 mL of 0.500M BaCl2
B)4.00 mL of 1.000M NaCl
C)7.50 mL of 0.500M FeCl3
D)25.00 mL of 0.400M KCl
E)30.00 mL of 0.100M CaCl2
A)10.0 mL of 0.500M BaCl2
B)4.00 mL of 1.000M NaCl
C)7.50 mL of 0.500M FeCl3
D)25.00 mL of 0.400M KCl
E)30.00 mL of 0.100M CaCl2

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
67
A 13.8 mL aliquot of 0.176 M H3PO4 (aq)is to be titrated with 0.110 M NaOH (aq). What volume (mL)of base will it take to reach the equivalence point?
A)7.29
B)22.1
C)199
D)66.2
E)20.9
A)7.29
B)22.1
C)199
D)66.2
E)20.9

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
68
What mass (g)of CaF2 is formed when 47.8 mL of 0.334 M NaF is treated with an excess of aqueous calcium nitrate?
A)1.25
B)0.472
C)2.49
D)0.943
E)0.623
A)1.25
B)0.472
C)2.49
D)0.943
E)0.623

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
69
What volume (L)of 0.250 M HNO3 is required to neutralize a solution prepared by dissolving 17.5 g of NaOH in 350 mL of water?
A)50.0
B)0.44
C)1.75
D)0.070
E)1.75 ×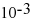
A)50.0
B)0.44
C)1.75
D)0.070
E)1.75 ×
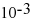

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
70
What mass (g)of potassium chloride is contained in 430.0 mL of a potassium chloride solution that has a chloride ion concentration of 0.193 M?
A)0.0643
B)0.0830
C)12.37
D)0.386
E)6.19
A)0.0643
B)0.0830
C)12.37
D)0.386
E)6.19

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
71
What volume (mL)of a concentrated solution of sodium hydroxide (6.00 M)must be diluted to 200.0 mL to make a 0.880 M solution of sodium hydroxide?
A)2.64
B)176
C)26.4
D)29.3
E)50.0
A)2.64
B)176
C)26.4
D)29.3
E)50.0

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
72
What volume (mL)of 7.48 × 10-2 M phosphoric acid can be neutralized with 115 mL of 0.244 M sodium hydroxide?
A)125
B)375
C)750
D)188
E)75.0
A)125
B)375
C)750
D)188
E)75.0

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
73
What volume (mL)of a concentrated solution of magnesium chloride (9.00 M)must be diluted to 350. mL to make a 2.75 M solution of magnesium chloride?
A)2.75
B)50.0
C)45.0
D)107
E)350
A)2.75
B)50.0
C)45.0
D)107
E)350

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
74
__________ is an oxidation reaction.
A)Ice melting in a soft drink
B)Table salt dissolving in water for cooking vegetables
C)Rusting of iron
D)The reaction of sodium chloride with lead nitrate to form lead chloride and sodium nitrate
E)Neutralization of HCl by NaOH
A)Ice melting in a soft drink
B)Table salt dissolving in water for cooking vegetables
C)Rusting of iron
D)The reaction of sodium chloride with lead nitrate to form lead chloride and sodium nitrate
E)Neutralization of HCl by NaOH

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
75
An aliquot (28.7 mL)of a KOH solution required 31.3 mL of 0.118 M HCl for neutralization. What mass (g)of KOH was in the original sample?
A)1.64
B)7.28
C)0.173
D)0.207
E)0.414
A)1.64
B)7.28
C)0.173
D)0.207
E)0.414

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
76
What volume (ml)of a 3.45 M lead nitrate solution must be diluted to 450.0 ml to make a 0.990 M solution of lead nitrate?
A)129
B)109
C)101
D)56
E)45
A)129
B)109
C)101
D)56
E)45

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
77
The point in a titration at which the indicator changes is called the __________.
A)setpoint
B)indicator point
C)standard point
D)endpoint
E)volumetric point
A)setpoint
B)indicator point
C)standard point
D)endpoint
E)volumetric point

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
78
What volume (mL)of 7.48 × 10-2 M perchloric acid can be neutralized with 115 mL of 0.244 M sodium hydroxide?
A)125
B)8.60
C)188
D)750
E)375
A)125
B)8.60
C)188
D)750
E)375

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
79
A 36.3 mL aliquot of 0.0529 M H2SO4 (aq)is to be titrated with 0.0411 M NaOH (aq). What volume (mL)of base will it take to reach the equivalence point?
A)93.4
B)46.7
C)187
D)1.92
E)3.84
A)93.4
B)46.7
C)187
D)1.92
E)3.84

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck
80
What volume (mL)of a concentrated solution of sodium hydroxide (6.00 M)must be diluted to 200. mL to make a 1.50 M solution of sodium hydroxide?
A)0.0500
B)50.0
C)45.0
D)800.
E)0.800
A)0.0500
B)50.0
C)45.0
D)800.
E)0.800

Unlock Deck
Unlock for access to all 177 flashcards in this deck.
Unlock Deck
k this deck


