Deck 6: Ionic Compounds: Periodic Trends and Bonding
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/138
Play
Full screen (f)
Deck 6: Ionic Compounds: Periodic Trends and Bonding
1
What is the ground-state electron configuration of the ion Cu2+?
A)[Ar] 3d9
B)[Ar] 4s1 3d8
C)[Ar] 4s2 3d7
D)[Ar] 4s2 3d10 4p1
A)[Ar] 3d9
B)[Ar] 4s1 3d8
C)[Ar] 4s2 3d7
D)[Ar] 4s2 3d10 4p1
[Ar] 3d9
2
Which of the following three sets consist of atoms or ions with the same electron configuration in the ground state? (I)O2-,Ne,and Mg2+
(II)Ni,Cu+,and Zn2+
(III)Hg,Tl+,and Pb2+
A)all three sets
B)(II)and (III)
C)(I)and (III)
D)(I)
(II)Ni,Cu+,and Zn2+
(III)Hg,Tl+,and Pb2+
A)all three sets
B)(II)and (III)
C)(I)and (III)
D)(I)
(I)and (III)
3
Arrange the ions N3-,O2-,Mg2+,Na+,and F- in order of increasing ionic radius,starting with the smallest first.
A)Mg2+,Na+,F-,O2-,N3-
B)N3-,Mg2+,O2-,Na+,F-
C)N3-,O2-,Mg2+,F-,Na+
D)N3-,O2-,F-,Na+,Mg2+
A)Mg2+,Na+,F-,O2-,N3-
B)N3-,Mg2+,O2-,Na+,F-
C)N3-,O2-,Mg2+,F-,Na+
D)N3-,O2-,F-,Na+,Mg2+
Mg2+,Na+,F-,O2-,N3-
4
Consider the following ground state electron configuration: 1s22s22p4.Which of the ions has this ground state electron configuration?
A)F-1
B)N+1
C)C-2
D)O-2
A)F-1
B)N+1
C)C-2
D)O-2

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
5
Of the following,which element has the highest first ionization energy?
A)P
B)C
C)Si
D)N
A)P
B)C
C)Si
D)N

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
6
Of the following,which element has the highest first ionization energy?
A)P
B)N
C)Sb
D)As
A)P
B)N
C)Sb
D)As

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
7
Which ion has the smallest ionic radius?
A)Li+
B)Na+
C)K+
D)Rb+
A)Li+
B)Na+
C)K+
D)Rb+

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
8
What is the ground-state electron configuration of Se2-?
A)[Ar]3d104s24p2
B)[Ar]3d104s24p4
C)[Ar]3d124s24p4
D)[Ar]3d104s24p6
A)[Ar]3d104s24p2
B)[Ar]3d104s24p4
C)[Ar]3d124s24p4
D)[Ar]3d104s24p6

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
9
Of the following,which element has the highest first ionization energy?
A)Cl
B)F
C)O
D)S
A)Cl
B)F
C)O
D)S

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
10
List the elements Na,Ca,Rb,Cl,He in order of decreasing first ionization energy.
A)He > Cl > Ca > Na > Rb
B)He > Na > Ca > Cl > Rb
C)He > Na > Cl > Ca > Rb
D)Rb > Ca > Cl > Na > He
A)He > Cl > Ca > Na > Rb
B)He > Na > Ca > Cl > Rb
C)He > Na > Cl > Ca > Rb
D)Rb > Ca > Cl > Na > He

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
11
Of the following,which element has the highest first ionization energy?
A)Li
B)F
C)Cs
D)At
A)Li
B)F
C)Cs
D)At

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
12
What is the ground-state electron configuration of the ion Hg2+?
A)[Xe]4f145d10
B)[Xe]4f145d86s2
C)[Xe]4f145d106s2
D)[Xe]4f145d106s26p2
A)[Xe]4f145d10
B)[Xe]4f145d86s2
C)[Xe]4f145d106s2
D)[Xe]4f145d106s26p2

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
13
Of the following,which element has the highest first ionization energy?
A)argon
B)neon
C)helium
D)krypton
A)argon
B)neon
C)helium
D)krypton

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
14
Of the following,which element has the highest first ionization energy?
A)iodine
B)bromine
C)fluorine
D)chlorine
A)iodine
B)bromine
C)fluorine
D)chlorine

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
15
List the elements Cs,Ca,Ne,Na,Ar in order of decreasing first ionization energy.
A)Ar > Ca > Cs > Na > Ne
B)Ne > Ar > Ca > Na > Cs
C)Ne > Ar > Na > Cs > Ca
D)Ne > Na > Cs > Ca > Ar
A)Ar > Ca > Cs > Na > Ne
B)Ne > Ar > Ca > Na > Cs
C)Ne > Ar > Na > Cs > Ca
D)Ne > Na > Cs > Ca > Ar

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
16
Which ion has the largest radius? Ca+2,Ca+1,Br-,K+
A)Ca+2
B)Ca+1
C)Br-
D)K+
A)Ca+2
B)Ca+1
C)Br-
D)K+

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
17
Which ion has the smallest ionic radius?
A)F-
B)Cl-
C)Br-
D)I-
A)F-
B)Cl-
C)Br-
D)I-

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following most likely represent the atomic radius of a Cr atom,the ionic radius of a Cr2+ ion,and the ionic radius of a Cr3+ ion?
A)128 pm for Cr,167 pm for Cr2+,and 193 pm for Cr3+
B)128 pm for Cr,147 pm for Cr2+,and 193 pm for Cr3+
C)128 pm for Cr,109 pm for Cr2+,and 63 pm for Cr3+
D)128 pm for Cr,89 pm for Cr2+,and 63 pm for Cr3+
A)128 pm for Cr,167 pm for Cr2+,and 193 pm for Cr3+
B)128 pm for Cr,147 pm for Cr2+,and 193 pm for Cr3+
C)128 pm for Cr,109 pm for Cr2+,and 63 pm for Cr3+
D)128 pm for Cr,89 pm for Cr2+,and 63 pm for Cr3+

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
19
Consider Li+,F-,and O2-.Which ratio should be the largest?
A)(radius Li+)/(radius F-)
B)(radius Li+)/(radius O2-)
C)(radius F-)/(radius Li+)
D)(radius O2-)/(radius Li+)
A)(radius Li+)/(radius F-)
B)(radius Li+)/(radius O2-)
C)(radius F-)/(radius Li+)
D)(radius O2-)/(radius Li+)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
20
Which ion has the same electron configuration as Kr?
A)Rb+
B)Br-
C)Se2-
D)All of these
A)Rb+
B)Br-
C)Se2-
D)All of these

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
21
Calculate the energy change for the formation of LiCl(s)from its elements in their standard states and the following tabulated information: Li(s)+ 1/2 Cl2(g)→ LiCl(s)?
Li+(g)+ Cl-(g)→ LiCl(s)-853 kJ/mol
Li(s)→ Li(g)+159.4 kJ/mol
1/2 Cl2(g)→ Cl(g)+121.7 kJ/mol
Cl(g)+ e- → Cl-(g)-348.6 kJ/mol
Li(g)→ Li+(g)+ e- +520.2 kJ/mol
A)+1305.7 kJ/mol
B)+296.9 kJ/mol
C)-400.3 kJ/mol
D)-627.2 kJ/mol
Li+(g)+ Cl-(g)→ LiCl(s)-853 kJ/mol
Li(s)→ Li(g)+159.4 kJ/mol
1/2 Cl2(g)→ Cl(g)+121.7 kJ/mol
Cl(g)+ e- → Cl-(g)-348.6 kJ/mol
Li(g)→ Li+(g)+ e- +520.2 kJ/mol
A)+1305.7 kJ/mol
B)+296.9 kJ/mol
C)-400.3 kJ/mol
D)-627.2 kJ/mol

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
22
Consider the following electron configurations for neutral atoms: atom I = 1s22s22p63s2
Atom II = 1s22s22p63s23p4
Atom III = 1s22s22p63s23p6
Which atom would be expected to have the largest third ionization energy?
A)atom I
B)atom II
C)atom III
D)All of these atoms would be expected to have the same third ionization energy.
Atom II = 1s22s22p63s23p4
Atom III = 1s22s22p63s23p6
Which atom would be expected to have the largest third ionization energy?
A)atom I
B)atom II
C)atom III
D)All of these atoms would be expected to have the same third ionization energy.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
23
Calculate the lattice energy for NaCl(s)using a Born-Haber cycle and the following information: NaCl(s)→ Na+(g)+ Cl-(g)?
Na(s)+ 1/2 Cl2(g)→ NaCl(s)-411.0 kJ/mol
Na(s)→ Na(g)+107.3 kJ/mol
Na(g)→ Na+(g)+ e- +495.8 kJ/mol
1/2 Cl2(g)→ Cl(g)+121.7 kJ/mol
Cl(g)+ e- → Cl-(g)-348.6 kJ/mol
A)+34.8 kJ/mol
B)+690.3 kJ/mol
C)+787.2 kJ/mol
D)+1512 kJ/mol
Na(s)+ 1/2 Cl2(g)→ NaCl(s)-411.0 kJ/mol
Na(s)→ Na(g)+107.3 kJ/mol
Na(g)→ Na+(g)+ e- +495.8 kJ/mol
1/2 Cl2(g)→ Cl(g)+121.7 kJ/mol
Cl(g)+ e- → Cl-(g)-348.6 kJ/mol
A)+34.8 kJ/mol
B)+690.3 kJ/mol
C)+787.2 kJ/mol
D)+1512 kJ/mol

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
24
Calculate the lattice energy for MgCl2(s)using a Born-Haber cycle and the following information: MgCl2(s)→ Mg2+(g)+ 2 Cl-(g)?
Mg(s)+ Cl2(g)→ MgCl2(s)-641.6 kJ/mol
Mg(s)→ Mg(g)+147.1 kJ/mol
Mg(g)→ Mg+(g)+ e- +737.8 kJ/mol
Mg+(g)→ Mg2+(g)+ e- +1451.0 kJ/mol
1/2 Cl2(g)→ Cl(g)+121.7 kJ/mol
Cl(g)+ e- → Cl-(g)-348.6 kJ/mol
A)+641.6 kJ/mol
B)+1240.5 kJ/mol
C)+1882.1 kJ/mol
D)+2523.7 kJ/mol
Mg(s)+ Cl2(g)→ MgCl2(s)-641.6 kJ/mol
Mg(s)→ Mg(g)+147.1 kJ/mol
Mg(g)→ Mg+(g)+ e- +737.8 kJ/mol
Mg+(g)→ Mg2+(g)+ e- +1451.0 kJ/mol
1/2 Cl2(g)→ Cl(g)+121.7 kJ/mol
Cl(g)+ e- → Cl-(g)-348.6 kJ/mol
A)+641.6 kJ/mol
B)+1240.5 kJ/mol
C)+1882.1 kJ/mol
D)+2523.7 kJ/mol

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
25
An element Y,has the following ionization energies Ei1 = 578 kJ/mol
Ei2 = 1,817 kJ/mol
Ei3 = 2,745 kJ/mol
Ei4 = 11,575 kJ/mol
What is the most likely identity for this element?
A)Na
B)Mg
C)Al
D)Si
Ei2 = 1,817 kJ/mol
Ei3 = 2,745 kJ/mol
Ei4 = 11,575 kJ/mol
What is the most likely identity for this element?
A)Na
B)Mg
C)Al
D)Si

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
26
Which ionization process requires the most energy?
A)Al(g)→ Al+(g)+ e-
B)Al+(g)→ Al2+(g)+ e-
C)Al2+(g)→ Al3+(g)+ e-
D)Al3+(g)→ Al4+(g)+ e-
A)Al(g)→ Al+(g)+ e-
B)Al+(g)→ Al2+(g)+ e-
C)Al2+(g)→ Al3+(g)+ e-
D)Al3+(g)→ Al4+(g)+ e-

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
27
In the reaction of sodium metal with chlorine gas which of the following processes releases energy?
A)Cl2(g)→ 2 Cl(g)
B)Cl(g)+ e- → Cl-(g)
C)Na(s)→ Na(g)
D)Na(g)→ Na+(g)+ e-
A)Cl2(g)→ 2 Cl(g)
B)Cl(g)+ e- → Cl-(g)
C)Na(s)→ Na(g)
D)Na(g)→ Na+(g)+ e-

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
28
What is the general trend in ionization energy and electron affinity values?
A)Both decrease as one traverses a period from left to right and both decrease as one descends a group.
B)Both decrease as one traverses a period from left to right and both increase as one descends a group.
C)Both increase as one traverses a period from left to right and both decrease as one descends a group.
D)Both increase as one traverses a period from left to right and both increase as one descends a group.
A)Both decrease as one traverses a period from left to right and both decrease as one descends a group.
B)Both decrease as one traverses a period from left to right and both increase as one descends a group.
C)Both increase as one traverses a period from left to right and both decrease as one descends a group.
D)Both increase as one traverses a period from left to right and both increase as one descends a group.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following atoms with the specified electronic configurations would have the lowest first ionization energy?
A)[He]2s22p3
B)[Ne]3s23p4
C)[Xe]6s1
D)[Xe]6s24f145d106p1
A)[He]2s22p3
B)[Ne]3s23p4
C)[Xe]6s1
D)[Xe]6s24f145d106p1

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
30
Which period 2 element has successive first through seventh ionization energies (kJ/mol)of Ei1 = 1,402;Ei2 = 2,856;Ei3 = 4,578;Ei4 = 7,475;Ei5 = 9,445;Ei6 = 53,267;and Ei7 = 64,360?
A)B
B)C
C)N
D)O
A)B
B)C
C)N
D)O

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
31
Which element has the most favorable (most negative)electron affinity?
A)K
B)Be
C)I
D)Ne
A)K
B)Be
C)I
D)Ne

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
32
Which of these elements has the most favorable (most negative)electron affinity?
A)Ca
B)N
C)Ne
D)S
A)Ca
B)N
C)Ne
D)S

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
33
An element that has the valence electron configuration 3s23p3 belongs to which period and group?
A)period 3;group 3A
B)period 3;group 5A
C)period 4;group 3A
D)period 4;group 5A
A)period 3;group 3A
B)period 3;group 5A
C)period 4;group 3A
D)period 4;group 5A

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
34
Which period 3 element has successive first through seventh ionization energies (kJ/mol)of Ei1 = 578;Ei2 = 1,817;Ei3 = 2,745;Ei4 = 11,575;Ei5 = 14,830;Ei6 = 18,376;and Ei7 = 23,293?
A)Mg
B)Al
C)S
D)Cl
A)Mg
B)Al
C)S
D)Cl

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
35
Which liberates the most energy?
A)Li(g)+ e- → Li-(g)
B)Na(g)+ e- → Na-(g)
C)K(g)+ e- → K-(g)
D)Rb(g)+ e- → Rb-(g)
A)Li(g)+ e- → Li-(g)
B)Na(g)+ e- → Na-(g)
C)K(g)+ e- → K-(g)
D)Rb(g)+ e- → Rb-(g)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following represents the change in electronic configuration that is associated with the second ionization energy of magnesium?
A)[Ne]3s2 → [Ne]3s1 + e-
B)[Ne]3s2 → [Ne] + 2 e-
C)[Ne]3s1 → [Ne] + e-
D)[Ne]3s2 + e- → [Ne]3s23p1
A)[Ne]3s2 → [Ne]3s1 + e-
B)[Ne]3s2 → [Ne] + 2 e-
C)[Ne]3s1 → [Ne] + e-
D)[Ne]3s2 + e- → [Ne]3s23p1

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
37
Which electron affinity process would liberate the most energy?
A)[He] 2s2 + e- → [He] 2s2 2p1
B)[He] 2s2 2p2 + e- → [He] 2s2 2p3
C)[He] 2s2 2p3 + e- → [He] 2s2 2p4
D)[He] 2s2 2p6 + e- → [He] 2s2 2p6 3s1
A)[He] 2s2 + e- → [He] 2s2 2p1
B)[He] 2s2 2p2 + e- → [He] 2s2 2p3
C)[He] 2s2 2p3 + e- → [He] 2s2 2p4
D)[He] 2s2 2p6 + e- → [He] 2s2 2p6 3s1

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
38
Element A has a valence shell configuration of ns2np4 while Element B has a valence shell configuration of ns2np5 where n = energy level.Which of the following statements is true regarding these elements?
A)The ionization energy of A is lower than the ionization energy of B.
B)The electron affinity of A is higher than the electron affinity of B.
C)Element A and B are transition metals.
D)Element A and B have the same I.E.
A)The ionization energy of A is lower than the ionization energy of B.
B)The electron affinity of A is higher than the electron affinity of B.
C)Element A and B are transition metals.
D)Element A and B have the same I.E.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
39
Which liberates the most energy?
A)Br(g)+ e⁻ → Br⁻(g)
B)Cl(g)+ e⁻ → Cl⁻(g)
C)F(g)+ e⁻ → F⁻(g)
D)I(g)→ I⁻(g)
A)Br(g)+ e⁻ → Br⁻(g)
B)Cl(g)+ e⁻ → Cl⁻(g)
C)F(g)+ e⁻ → F⁻(g)
D)I(g)→ I⁻(g)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
40
Which element has the most favorable (most negative)electron affinity?
A)B
B)C
C)Li
D)N
A)B
B)C
C)Li
D)N

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
41
Calculate the electron affinity for the formation of the hydride ion from the following information: H(g)+ e- → H-(g)?
1/2 H2(g)→ H(g)+217.9 kJ/mol
Na(s)→ Na(g)+107.3 kJ/mol
Na(g)→ Na+(g)+ e- +495.8 kJ/mol
Na(s)+ 1/2 H2(g)→ NaH(s)-60.0 kJ/mol
NaH(s)→ Na+(g)+ H-(g)+810.9 kJ/mol
A)-50.1 kJ/mol
B)-70.1 kJ/mol
C)-816 kJ/mol
D)-1632 kJ/mol
1/2 H2(g)→ H(g)+217.9 kJ/mol
Na(s)→ Na(g)+107.3 kJ/mol
Na(g)→ Na+(g)+ e- +495.8 kJ/mol
Na(s)+ 1/2 H2(g)→ NaH(s)-60.0 kJ/mol
NaH(s)→ Na+(g)+ H-(g)+810.9 kJ/mol
A)-50.1 kJ/mol
B)-70.1 kJ/mol
C)-816 kJ/mol
D)-1632 kJ/mol

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
42
The four spheres below represent Na+,Mg2+,F⁻,and O2-,not necessarily in that order. 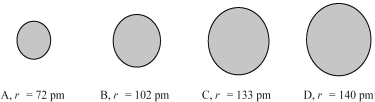
Which sphere most likely represents the Na⁺ ion?
A)A
B)B
C)C
D)D

Which sphere most likely represents the Na⁺ ion?
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
43
Which chemical process is associated with the lattice energy for sodium chloride?
A)NaCl(s)→ Na+(g)+ Cl-(g)
B)NaCl(g)→ Na+(g)+ Cl-(g)
C)Na(s)+ 1/2 Cl2(g)→ NaCl(s)
D)NaCl(s)+ H2O(l)→ Na+(aq)+ Cl-(aq)
A)NaCl(s)→ Na+(g)+ Cl-(g)
B)NaCl(g)→ Na+(g)+ Cl-(g)
C)Na(s)+ 1/2 Cl2(g)→ NaCl(s)
D)NaCl(s)+ H2O(l)→ Na+(aq)+ Cl-(aq)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
44
The following four spheres represent an Mg atom,an Mg2+ ion,a S atom,and a S2- ion,not necessarily in that order.Use your knowledge about the relative sizes of atoms,cations,and anions to determine which of the following sets of reactions is most consistent with the sizes of the atoms and ions shown below. 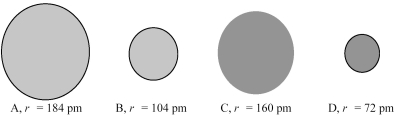
A)A → B + 2e⁻ and C → D + 2e⁻
B)A → B + 2e⁻ and D → C + 2e⁻
C)B → A + 2e⁻ and C → D + 2e⁻
D)B → A + 2e⁻ and D → C + 2e⁻

A)A → B + 2e⁻ and C → D + 2e⁻
B)A → B + 2e⁻ and D → C + 2e⁻
C)B → A + 2e⁻ and C → D + 2e⁻
D)B → A + 2e⁻ and D → C + 2e⁻

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
45
Which ionic compound would be expected to have the highest lattice energy?
A)Li2O
B)Na2O2
C)KO2
D)RbO2
A)Li2O
B)Na2O2
C)KO2
D)RbO2

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
46
The following four spheres represent a metal atom,a nonmetal atom,a monatomic anion and a monatomic cation,not necessarily in that order. 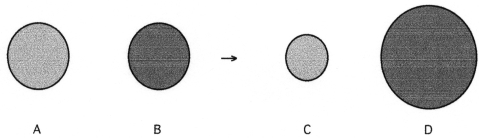
Which sphere represents the monatomic anion?
A)A
B)B
C)C
D)D

Which sphere represents the monatomic anion?
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
47
The following four spheres represent a metal atom,a nonmetal atom,a monatomic anion and a monatomic cation,not necessarily in that order. 
Which sphere represents the metal atom?
A)A
B)B
C)C
D)D

Which sphere represents the metal atom?
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
48
Calculate the lattice energy for MgO(s)using a Born-Haber cycle and the following information: MgO(s)→ Mg2+(g)+ O2-(g)?
Mg(s)→ Mg(g)+147.1 kJ/mol
Mg(g)→ Mg+(g)+ e- +737.8 kJ/mol
Mg+(g)→ Mg2+(g)+ e- +1451 kJ/mol
1/2 O2(g)→ O(g)+249.0 kJ/mol
O(g)+ e- → O-(g)-141.1 kJ/mol
O-(g)+ e- → O2-(g)+798.0 kJ/mol
Mg(s)+ 1/2 O2(g)→ MgO(s)-601.8 kJ/mol
A)+1842 kJ/mol
B)+2444 kJ/mol
C)+3844 kJ/mol
D)+4108 kJ/mol
Mg(s)→ Mg(g)+147.1 kJ/mol
Mg(g)→ Mg+(g)+ e- +737.8 kJ/mol
Mg+(g)→ Mg2+(g)+ e- +1451 kJ/mol
1/2 O2(g)→ O(g)+249.0 kJ/mol
O(g)+ e- → O-(g)-141.1 kJ/mol
O-(g)+ e- → O2-(g)+798.0 kJ/mol
Mg(s)+ 1/2 O2(g)→ MgO(s)-601.8 kJ/mol
A)+1842 kJ/mol
B)+2444 kJ/mol
C)+3844 kJ/mol
D)+4108 kJ/mol

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
49
Calculate the energy change in kJ/mol for the reaction 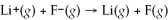 using the following information: Li(g)→ Li+(g)+ e- +520 kJ/mol
using the following information: Li(g)→ Li+(g)+ e- +520 kJ/mol
F(g)+ e- → F-(g)-328 kJ/mol
A)-848 kJ/mol
B)-192 kJ/mol
C)+192 kJ/mol
D)+848 kJ/mol
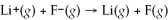 using the following information: Li(g)→ Li+(g)+ e- +520 kJ/mol
using the following information: Li(g)→ Li+(g)+ e- +520 kJ/molF(g)+ e- → F-(g)-328 kJ/mol
A)-848 kJ/mol
B)-192 kJ/mol
C)+192 kJ/mol
D)+848 kJ/mol

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
50
The four spheres below represent Na+,Mg2+,F⁻,and O2-,not necessarily in that order. 
Which sphere most likely represents the F- ion?
A)A
B)B
C)C
D)D

Which sphere most likely represents the F- ion?
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
51
The following four spheres represent a metal atom,a nonmetal atom,a monatomic anion and a monatomic cation,not necessarily in that order. 
Which sphere represents the nonmetal atom?
A)A
B)B
C)C
D)D

Which sphere represents the nonmetal atom?
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
52
The following four spheres represent a Na atom,a Na+ ion,a Cl atom,and a Cl- ion,not necessarily in that order.Use your knowledge about the relative sizes of atoms,cations,and anions to determine which of the following sets of reactions is most consistent with the sizes of the atoms and ions shown below. 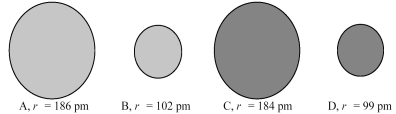
A)A → B + e- and C → D + e-
B)A → B + e- and D → C + e-
C)B → A + e- and C → D + e-
D)B → A + e- and D → C + e-

A)A → B + e- and C → D + e-
B)A → B + e- and D → C + e-
C)B → A + e- and C → D + e-
D)B → A + e- and D → C + e-

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
53
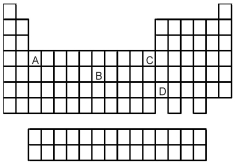
Which element,indicated by letter on the periodic table above,has a 1+ ion with the electron configuration
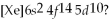
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
54

Which element,indicated by letter on the periodic table above,has a 3+ ion with the electron configuration
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
55
Calculate the energy change for the formation of CaF2(s)from its elements in their standard states and the following information: Ca(s)+ F2(g)→ CaF2(s)?
Ca(s)→ Ca(g)+179.3 kJ/mol
Ca(g)→ Ca+(g)+ e- +589.9 kJ/mol
Ca+(g)→ Ca2+(g)+ e- +1145 kJ/mol
1/2 F2(g)→ F(g)+79.0 kJ/mol
F(g)+ e- → F-(g)-328.0 kJ/mol
CaF2(s)→ Ca2+(g)+ 2 F-(g)+2630 kJ/mol
A)+4046 kJ/mol
B)-965 kJ/mol
C)-1214 kJ/mol
D)-3286 kJ/mol
Ca(s)→ Ca(g)+179.3 kJ/mol
Ca(g)→ Ca+(g)+ e- +589.9 kJ/mol
Ca+(g)→ Ca2+(g)+ e- +1145 kJ/mol
1/2 F2(g)→ F(g)+79.0 kJ/mol
F(g)+ e- → F-(g)-328.0 kJ/mol
CaF2(s)→ Ca2+(g)+ 2 F-(g)+2630 kJ/mol
A)+4046 kJ/mol
B)-965 kJ/mol
C)-1214 kJ/mol
D)-3286 kJ/mol

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
56
The following four spheres represent a metal atom,a nonmetal atom,a monatomic anion and a monatomic cation,not necessarily in that order. 
Which sphere represents the monatomic cation?
A)A
B)B
C)C
D)D

Which sphere represents the monatomic cation?
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
57
![<strong> Which element,indicated by letter on the periodic table above,has a 2+ ion with the electron configuration [Ar]3d<sup>10</sup>?</strong> A)A B)B C)C D)D](https://storage.examlex.com/TB4940/11ea7e2d_d0bc_4965_a2f7_cbf1237fd3a7_TB4940_00_TB4940_00_TB4940_00_TB4940_00.jpg)
Which element,indicated by letter on the periodic table above,has a 2+ ion with the electron configuration [Ar]3d10?
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
58
![<strong> Which element,indicated by letter on the periodic table above,has a 3+ ion with the electron configuration [Kr]4d<sup>5</sup>?</strong> A)A B)B C)C D)D](https://storage.examlex.com/TB4940/11ea7e2d_d0bc_4965_a2f7_cbf1237fd3a7_TB4940_00_TB4940_00_TB4940_00_TB4940_00.jpg)
Which element,indicated by letter on the periodic table above,has a 3+ ion with the electron configuration [Kr]4d5?
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
59
The four spheres below represent Na+,Mg2+,F⁻,and O2-,not necessarily in that order. 
Which sphere most likely represents the Mg2+ ion?
A)A
B)B
C)C
D)D

Which sphere most likely represents the Mg2+ ion?
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
60
Calculate the energy change for the formation of MgBr2(s)from its elements in their standard states: Mg(s)+ Br2(l)→ MgBr2(s)?
Mg(s)→ Mg(g)+147.1 kJ/mol
Mg(g)→ Mg+(g)+ e- +737.8 kJ/mol
Mg+(g)→ Mg2+(g)+ e- +1451 kJ/mol
Br2(l)→ Br2(g)+30.9 kJ/mol
1/2 Br2(g)→ Br(g)+112.0 kJ/mol
Br(g)+ e- → Br-(g)-325.0 kJ/mol
MgBr2(s)→ Mg2+(g)+ 2Br-(g)+2440 kJ/mol
A)-150.8 kJ/mol
B)-286.0 kJ/mol
C)-499.2 kJ/mol
D)-5682 kJ/mol
Mg(s)→ Mg(g)+147.1 kJ/mol
Mg(g)→ Mg+(g)+ e- +737.8 kJ/mol
Mg+(g)→ Mg2+(g)+ e- +1451 kJ/mol
Br2(l)→ Br2(g)+30.9 kJ/mol
1/2 Br2(g)→ Br(g)+112.0 kJ/mol
Br(g)+ e- → Br-(g)-325.0 kJ/mol
MgBr2(s)→ Mg2+(g)+ 2Br-(g)+2440 kJ/mol
A)-150.8 kJ/mol
B)-286.0 kJ/mol
C)-499.2 kJ/mol
D)-5682 kJ/mol

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
61
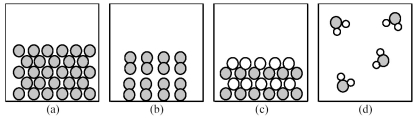
Which of the above pictures best represents a solid ionic compound?
A)picture (a)
B)picture (b)
C)picture (c)
D)picture (d)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
62
The four spheres below represent K+,Ca2+,Cl-,and S2-,not necessarily in that order. 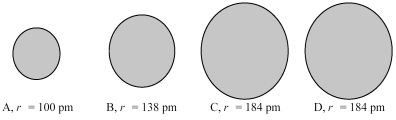
Which sphere most likely represents the K+ ion?
A)A
B)B
C)A or B
D)C or D

Which sphere most likely represents the K+ ion?
A)A
B)B
C)A or B
D)C or D

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
63
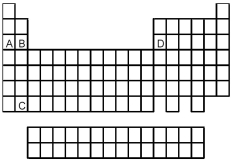
Atoms of which element,indicated by letter on the periodic table above,would be expected to have the highest second ionization energy,Ei1?
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
64
The four spheres below represent Na+,Mg2+,F⁻,and O2-,not necessarily in that order. 
Which sphere most likely represents the O2- ion?
A)A
B)B
C)C
D)D

Which sphere most likely represents the O2- ion?
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
65

Atoms of which element,indicated by letter on the periodic table,would be expected to have the most negative value of Eea?
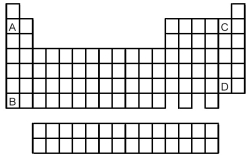
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
66

Atoms of which element,indicated by letter on the periodic table above,would be expected to have the lowest second ionization energy,Ei1?
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
67
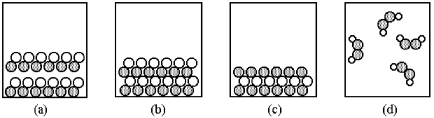
Which of the above pictures are more likely to represent covalent compounds?
A)pictures (a)and (b)
B)pictures (a)and (d)
C)pictures (b)and (c)
D)pictures (b)and (d)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
68
The four spheres below represent K+,Ca2+,Cl-,and S2-,not necessarily in that order. 
Which sphere most likely represents the Ca2+ ion?
A)A
B)B
C)A or B
D)C or D

Which sphere most likely represents the Ca2+ ion?
A)A
B)B
C)A or B
D)C or D

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
69
The four spheres below represent K+,Ca2+,Cl-,and S2-,not necessarily in that order. 
Which sphere most likely represents the Cl⁻ ion?
A)A
B)B
C)A or B
D)C or D

Which sphere most likely represents the Cl⁻ ion?
A)A
B)B
C)A or B
D)C or D

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
70
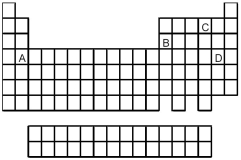
What is the likely formula for the binary compound formed from the elements represented by letters A and D on the periodic table above?
A)AD
B)A2D
C)AD2
D)A2D3

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
71

Which of the above pictures best represents a gaseous covalent compound?
A)picture (a)
B)picture (b)
C)picture (c)
D)picture (d)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
72
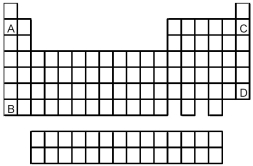
Atoms of which element,indicated by letter on the periodic table above,would be expected to have the highest first ionization energy,Ei1?
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
73

What is the likely formula for the binary compound formed from the elements represented by letters A and C on the periodic table above?
A)AC
B)A2C
C)AC2
D)A2C3

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
74
The following pictures represent alkali halide salts. 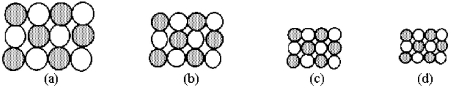
Which salt has the highest lattice energy?
A)picture (a)
B)picture (b)
C)picture (c)
D)picture (d)

Which salt has the highest lattice energy?
A)picture (a)
B)picture (b)
C)picture (c)
D)picture (d)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
75
The four spheres below represent K+,Ca2+,Cl-,and S2-,not necessarily in that order. 
Which sphere most likely represents the S2- ion?
A)A
B)B
C)A or B
D)C or D

Which sphere most likely represents the S2- ion?
A)A
B)B
C)A or B
D)C or D

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
76
The following pictures represent alkali halide salts. 
Which salt has the lowest lattice energy?
A)picture (a)
B)picture (b)
C)picture (c)
D)picture (d)

Which salt has the lowest lattice energy?
A)picture (a)
B)picture (b)
C)picture (c)
D)picture (d)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
77
Which contains ionic bonds?
A)CH4
B)CaCl2
C)Cl2
D)NCl3
A)CH4
B)CaCl2
C)Cl2
D)NCl3

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
78
Which element,indicated by letter on the periodic table,is able to form compounds that do not obey the octet rule? 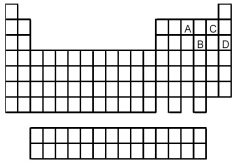
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
79

Atoms of which element,indicated by letter on the periodic table above,would be expected to have the lowest first ionization energy,Ei1?
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
80

Which of the above pictures are more likely to represent ionic compounds?
A)pictures (a)and (b)
B)pictures (a)and (d)
C)pictures (b)and (c)
D)pictures (b)and (d)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck


