Deck 16: Applications of Aqueous Equilibria
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/201
Play
Full screen (f)
Deck 16: Applications of Aqueous Equilibria
1
What volume of 0.100 M NaOH is needed to make 100.0 mL of a buffer solution with a pH of 6.00 if one starts with 50.0 mL of 0.100 M potassium hydrogen phthalate? The Ka2 for potassium hydrogen phthalate is 3.1 × 10-6.
A)22.4 mL
B)27.6 mL
C)30.2 mL
D)37.8 mL
A)22.4 mL
B)27.6 mL
C)30.2 mL
D)37.8 mL
37.8 mL
2
What is the pH of a solution prepared by mixing 50.00 mL of 0.10 M NH3 with 25.00 mL of 0.10 M NH4Cl? Assume that the volume of the solutions are additive and that Kb = 1.8 × 10-5 for NH3.
A)8.95
B)9.26
C)9.56
D)11.13
A)8.95
B)9.26
C)9.56
D)11.13
9.56
3
What is the approximate value of the equilibrium constant,Kn,for the neutralization of nitrous acid with ammonia,shown in the equation below? The Ka for HNO2 is 4.5 × 10-4 and the Kb for NH3 is 1.8 × 10-5. HNO2(aq)+ NH3(aq)⇌ NH4NO2(aq)
A)8.1 × 105
B)1.8 × 109
C)4.5 × 1010
D)8.1 × 1019
A)8.1 × 105
B)1.8 × 109
C)4.5 × 1010
D)8.1 × 1019
8.1 × 105
4
What is the pH of a buffer solution made by mixing 50.0 mL of 0.100 M potassium hydrogen phthalate with 13.6 mL of 0.100 M NaOH and diluting the mixture to 100.0 mL with water? The Ka2 for hydrogen phthalate is 3.1 × 10-6.
A)3.25
B)5.08
C)5.51
D)5.94
A)3.25
B)5.08
C)5.51
D)5.94

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
5
What is the pH of a buffered system made by dissolving 17.42 g of KH2PO4 and 20.41 g of K2HPO4 in water to give a volume of 200.0 mL? The Ka2 for dihydrogen phosphate is 6.2 × 10-8 and the equilibrium reaction of interest is H2PO4-(aq)+ H2O(l)⇌ H3O+(aq)+ HPO4-(aq).
A)7.03
B)7.17
C)7.38
D)7.58
A)7.03
B)7.17
C)7.38
D)7.58

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
6
TRIS {(HOCH2)3CNH2} is one of the most common buffers used in biochemistry.A solution is prepared by adding enough TRIS and 12 M HCl(aq)to give 1.00 L of solution with [TRIS] = 0.30 M and [TRISH+] = 0.60 M.What is the pH of this buffered system if the pKb is 5.92?
A)5.92
B)6.22
C)7.78
D)8.08
A)5.92
B)6.22
C)7.78
D)8.08

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
7
What is the hydronium ion concentration in a solution prepared by mixing 50.00 mL of 0.10 M HCN with 50.00 mL of  NaCN? Assume that the volumes of the solutions are additive and that Ka = 4.9 × 10-10 for HCN.
NaCN? Assume that the volumes of the solutions are additive and that Ka = 4.9 × 10-10 for HCN.
A)2.4 × 10-10 M
B)4.9 × 10-10 M
C)9.8 × 10-10 M
D)7.0 × 10-6 M
 NaCN? Assume that the volumes of the solutions are additive and that Ka = 4.9 × 10-10 for HCN.
NaCN? Assume that the volumes of the solutions are additive and that Ka = 4.9 × 10-10 for HCN.A)2.4 × 10-10 M
B)4.9 × 10-10 M
C)9.8 × 10-10 M
D)7.0 × 10-6 M

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
8
What is the approximate value of the equilibrium constant,Kn,for the neutralization of pyridine with hydrochloric acid,shown in the equation below? The Kb for pyridine is 1.8 × 10-9. HCl(aq)+ C5H5N(aq)⇌ C5H5NHCl(aq)
A)5.6 × 10-10
B)5.6 × 10-6
C)1.8 × 105
D)5.6 × 108
A)5.6 × 10-10
B)5.6 × 10-6
C)1.8 × 105
D)5.6 × 108

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
9
What is the pH of a buffer system made by dissolving 10.70 grams of NH4Cl and 20.00 mL of 12.0 M NH3 in enough water to make 1.000 L of solution? Kb = 1.8 × 10-5 for NH3.
A)9.18
B)9.26
C)9.34
D)11.03
A)9.18
B)9.26
C)9.34
D)11.03

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
10
Which statement about buffers is true?
A)Buffers have a pH = 7.
B)Buffers consist of a strong acid and its conjugate base.
C)A buffer does not change pH on addition of a strong acid or strong base.
D)Buffers resist change in pH upon addition of small amounts of strong acid or strong base.
A)Buffers have a pH = 7.
B)Buffers consist of a strong acid and its conjugate base.
C)A buffer does not change pH on addition of a strong acid or strong base.
D)Buffers resist change in pH upon addition of small amounts of strong acid or strong base.

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
11
The neutralization constant Kn for the neutralization of acetylsalicylic acid (C9H8O4)and codeine (C18H21NO3)is 4.8 × 104.The acid dissociation constant Ka for acetylsalicylic acid is 3.0 × 10-4.What is the base dissociation constant Kb for codeine?
A)4.8 × 10-24
B)6.3 × 10-23
C)1.6 × 10-6
D)1.4 × 1015
A)4.8 × 10-24
B)6.3 × 10-23
C)1.6 × 10-6
D)1.4 × 1015

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
12
The neutralization constant Kn for the neutralization of phenobarbital (C12H12N2O3)and morphine (C17H19NO3)is 2.9.The acid dissociation constant Ka for phenobarbital is 3.9 × 10-8.What is the base dissociation constant Kb for morphine?
A)1.3 × 10-22
B)6.3 × 10-23
C)7.4 × 10-7
D)1.1 × 107
A)1.3 × 10-22
B)6.3 × 10-23
C)7.4 × 10-7
D)1.1 × 107

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
13
A buffer solution is prepared by dissolving 0.200 mol of NaH2PO4 and 0.100 mol of NaOH in enough water to make 1.00 L of solution.What is the pH of the H2PO4-/HPO42- buffer if the Ka2 = 6.2 × 10-8?
A)6.91
B)7.21
C)7.51
D)7.71
A)6.91
B)7.21
C)7.51
D)7.71

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
14
What is the [CH3CO2-]/[CH3CO2H] ratio necessary to make a buffer solution with a pH of 4.44? Ka = 1.8 × 10-5 for CH3CO2H.
A)0.50:1
B)0.94:1
C)1.1:1
D)2.0:1
A)0.50:1
B)0.94:1
C)1.1:1
D)2.0:1

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
15
What is the pH of a solution prepared by mixing 25.00 mL of 0.10 M methylamine,CH3NH2,with 25.00 mL of 0.10 M methylammonium chloride,CH3NH3Cl? Assume that the volume of the solutions are additive and that Kb = 3.70 × 10-4 for methylamine.
A)10.27
B)10.57
C)10.87
D)11.78
A)10.27
B)10.57
C)10.87
D)11.78

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
16
What is the pH of 1 L of 0.30 M TRIS,0.60 M TRISH+ buffer to which one has added 5.0 mL of 12 M HCl? The Kb for the TRIS/TRISH+ is 1.2 × 10-6.
A)5.92
B)6.36
C)7.36
D)7.64
A)5.92
B)6.36
C)7.36
D)7.64

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
17
What is the resulting pH when 0.005 moles of KOH is added to 0.100 L of a buffer solution that is 0.100 M in H2PO4- and 0.100 M HPO42- and the Ka2 = 6.2 × 10-8?
A)5.21
B)5.61
C)6.73
D)7.69
A)5.21
B)5.61
C)6.73
D)7.69

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
18
What is the pH of a solution prepared by mixing 25.00 mL of 0.10 M CH3CO2H with 25.00 mL of 0.050 M CH3CO2Na? Assume that the volume of the solutions are additive and that Ka = 1.8 × 10-5 for CH3CO2H.
A)2.87
B)4.44
C)4.74
D)5.05
A)2.87
B)4.44
C)4.74
D)5.05

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
19
A buffer solution is prepared by dissolving 27.22 g of KH2PO4 and 3.37 g of KOH in enough water to make 0.100 L of solution.What is the pH of the H2PO4-/HPO42- buffer if the Ka2 = 6.2 × 10-8?
A)6.84
B)7.00
C)7.21
D)7.84
A)6.84
B)7.00
C)7.21
D)7.84

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
20
What is the magnitude of the change in pH when 0.005 moles of HCl is added to 0.100 L of a buffer solution that is 0.100 M in CH3CO2H and 0.100 M NaCH3CO2? The Ka for acetic acid is 1.8 × 10-5.
A)0.00
B)0.20
C)0.47
D)1.30
A)0.00
B)0.20
C)0.47
D)1.30

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
21
What is the Henderson-Hasselbalch equation for the acidic buffer HA/A-?
A)pH = -log[H3O+]
B)pH = 14 - pOH
C)pH = pKa + log{[A-]/[HA]}
D)pH = pKa - log{[A-]/[HA]}
A)pH = -log[H3O+]
B)pH = 14 - pOH
C)pH = pKa + log{[A-]/[HA]}
D)pH = pKa - log{[A-]/[HA]}

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following buffer solutions will exhibit the highest pH?
A)0.100 M NH3 with 0.100 M NH4+
B)0.200 M NH3 with 0.100 M NH4+
C)0.200 M NH3 with 0.0400M NH4+
D)0.800 M NH3 with 0.800 M NH4+
A)0.100 M NH3 with 0.100 M NH4+
B)0.200 M NH3 with 0.100 M NH4+
C)0.200 M NH3 with 0.0400M NH4+
D)0.800 M NH3 with 0.800 M NH4+

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
23
What is the approximate pH at the equivalence point of a weak acid-strong base titration if 25 mL of aqueous formic acid requires 29.80 mL of 0.0567 M NaOH? Ka = 1.8 × 10-4 for formic acid.
A)2.46
B)5.88
C)8.12
D)11.54
A)2.46
B)5.88
C)8.12
D)11.54

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
24
Formic acid (HCO2H,Ka = 1.8 × 10-4)is the principal component in the venom of stinging ants.What is the molarity of a formic acid solution if 25.00 mL of the formic acid solution requires 29.80 mL of 0.0567 M NaOH to reach the equivalence point?
A)0.0134 M
B)0.0476 M
C)0.0567 M
D)0.0676 M
A)0.0134 M
B)0.0476 M
C)0.0567 M
D)0.0676 M

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
25
What is the approximate pH at the equivalence point of a weak acid-strong base titration if 25 mL of aqueous hydrofluoric acid requires 30.00 mL of 0.400 M NaOH? Ka = 6.76 × 10-4 for HF.
A)1.74
B)5.75
C)8.25
D)12.26
A)1.74
B)5.75
C)8.25
D)12.26

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
26
Consider a buffered solution consisting of H2CO3 and HCO3- where the pKa = 6.4.At pH = 6.4,which of the following is true?
A)[H2CO3] > [HCO3-]
B)[H2CO3]< [HCO3-]
C)[H2CO3] = [HCO3-]
D)[H2CO3] < [CO3-2]
A)[H2CO3] > [HCO3-]
B)[H2CO3]< [HCO3-]
C)[H2CO3] = [HCO3-]
D)[H2CO3] < [CO3-2]

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following mixtures would result in a buffered solution when 1.0 L of each of the two solutions are mixed.
A)0.2 M HNO3 and 0.2 M NaNO3
B)0.2 M HNO3 and 0.4 M HF
C)0.2 M HNO3 and 0.4 M NaF
D)0.2 M HNO3 and 0.4 M NaOH
A)0.2 M HNO3 and 0.2 M NaNO3
B)0.2 M HNO3 and 0.4 M HF
C)0.2 M HNO3 and 0.4 M NaF
D)0.2 M HNO3 and 0.4 M NaOH

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
28
At what pH is the amino acid glycine with a Ka of 2.51 × 10-10 sixty-six (66%)percent dissociated?
A)9.60
B)9.89
C)10.10
D)10.60
A)9.60
B)9.89
C)10.10
D)10.60

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
29
What is the pH of a solution made by mixing 30.00 mL of 0.10 M acetic acid with 40.00 mL of 0.10 M KOH? Assume that the volumes of the solutions are additive.Ka = 1.8 × 10-5 for CH3CO2H.
A)8.26
B)9.26
C)11.13
D)12.15
A)8.26
B)9.26
C)11.13
D)12.15

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
30
What is the pH at the equivalence point of a weak acid-strong base titration?
A)pH < 7
B)pH = 7
C)pH > 7
D)pH = 14.00
A)pH < 7
B)pH = 7
C)pH > 7
D)pH = 14.00

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
31
What is not a correct expression for the weak acid HA?
A)Ka = [H3O+][A-]/[HA]
B)pKa = pH - log{[A-]/[HA]}
C)pKa = logKa
D)pKa = 14 - pKb
A)Ka = [H3O+][A-]/[HA]
B)pKa = pH - log{[A-]/[HA]}
C)pKa = logKa
D)pKa = 14 - pKb

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
32
What volume of 5.00 × 10-3 M HNO3 is needed to titrate 100.00 mL of 5.00 × 10-3 M Ca(OH)2 to the equivalence point?
A)12.5 mL
B)50.0 mL
C)100.mL
D)200.mL
A)12.5 mL
B)50.0 mL
C)100.mL
D)200.mL

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
33
What is the pH of a solution made by mixing 30.00 mL of 0.10 M HCl with 40.00 mL of 0.10 M KOH? Assume that the volumes of the solutions are additive.
A)0.85
B)1.85
C)12.15
D)13.15
A)0.85
B)1.85
C)12.15
D)13.15

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
34
Which is the best acid to use in the preparation of a buffer with pH = 3.3?
A)HOI (Ka = 2.0 × 10-11)
B)HNO2 (Ka = 4.5 × 10-4)
C)HNO3
D)HIO3 (Ka = 1.7 × 10-1)
A)HOI (Ka = 2.0 × 10-11)
B)HNO2 (Ka = 4.5 × 10-4)
C)HNO3
D)HIO3 (Ka = 1.7 × 10-1)

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
35
What is the pH of the resulting solution if 30.00 mL of 0.10 M acetic acid is added to 10.00 mL of 0.10 M NaOH? Assume that the volumes of the solutions are additive.Ka = 1.8 × 10-5 for CH3CO2H.
A)9.56
B)8.95
C)5.05
D)4.44
A)9.56
B)8.95
C)5.05
D)4.44

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
36
What is the percent dissociation of ascorbic acid if the solution has a pH = 5.50 and a pKa = 4.10?
A)96%
B)10%
C)5%
D)1%
A)96%
B)10%
C)5%
D)1%

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
37
Which is the best acid to use in the preparation of a buffer with pH = 9.3?
A)CH3NH2 (Kb = 3.7 × 10-4)
B)NH3 (Kb = 1.8 × 10-5)
C)NH2OH (Kb = 9.1 × 10-9)
D)C6H5NH2 (Kb = 4.3 × 10-10)
A)CH3NH2 (Kb = 3.7 × 10-4)
B)NH3 (Kb = 1.8 × 10-5)
C)NH2OH (Kb = 9.1 × 10-9)
D)C6H5NH2 (Kb = 4.3 × 10-10)

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
38
What is the percent dissociation of acetic acid if the solution has a pH = 4.74 and a pKa = 4.74?
A)100%
B)50%
C)10%
D)1%
A)100%
B)50%
C)10%
D)1%

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
39
What is the percent dissociation of glycine if the solution has a pH = 8.60 and a pKa = 9.60?
A)50%
B)9%
C)5%
D)1%
A)50%
B)9%
C)5%
D)1%

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
40
What is the pH of a solution made by mixing 30.00 mL of 0.10 M acetic acid with 30.00 mL of 0.10 M KOH? Assume that the volumes of the solutions are additive.Ka = 1.8 × 10-5 for CH3CO2H.
A)5.28
B)7.00
C)8.72
D)10.02
A)5.28
B)7.00
C)8.72
D)10.02

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
41
What is the molar solubility of AgCl in 0.10 M NH3? Ksp for AgCl is 1.8 × 10-10 and the Kf for Ag(NH3)2+ is 1.7 × 107.
A)1.3 × 10-5 M
B)5.0 × 10-3 M
C)5.5 × 10-3 M
D)5.5 × 10-2 M
A)1.3 × 10-5 M
B)5.0 × 10-3 M
C)5.5 × 10-3 M
D)5.5 × 10-2 M

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
42
Sodium hypochlorite,NaOCl,is the active ingredient in household bleach.What is the concentration of hypochlorite ion if 20.00 mL of bleach requires 28.30 mL of 0.500 M HCl to reach the equivalence point?
A)0.208 M
B)0.353 M
C)0.708 M
D)1.21 M
A)0.208 M
B)0.353 M
C)0.708 M
D)1.21 M

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
43
Calculate the molar solubility of thallium(I)chloride in 0.30 M NaCl at 25°C.Ksp for TlCl is 1.7 × 10-4.
A)5.1 × 10-5 M
B)5.7 × 10-4 M
C)7.1 × 10-3 M
D)1.3 × 10-2 M
A)5.1 × 10-5 M
B)5.7 × 10-4 M
C)7.1 × 10-3 M
D)1.3 × 10-2 M

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
44
The dissociation equilibrium constants for the protonated form of alanine (a diprotic amino acid H2X+)are Ka1 = 4.6 × 10-3 and Ka2 = 2.0 × 10-10.What is the pH of 50.00 mL of a 0.100 M solution of alanine after 100.00 mL of 0.100 M NaOH has been added?
A)9.70
B)10.69
C)11.11
D)12.70
A)9.70
B)10.69
C)11.11
D)12.70

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
45
Barium hydroxide is slightly soluble in water,with a Ksp of 5.00 × 10-4 at 298K.The dissolution of barium hydroxide in water is an endothermic process. Ba+2 (aq)+ 2OH- (aq)
Which of the following will increase the solubility?
A)Barium hydroxide is added to the solution.
B)Sodium hydroxide (NaOH)is added to the solution.
C)The temperature is decreased.
D)HCl is added to the mixture.(HCl reacts with OH-,removing it from the system. )
Which of the following will increase the solubility?
A)Barium hydroxide is added to the solution.
B)Sodium hydroxide (NaOH)is added to the solution.
C)The temperature is decreased.
D)HCl is added to the mixture.(HCl reacts with OH-,removing it from the system. )

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
46
What is the molar solubility of AgCl in 0.10 M NaCN if the colorless complex ion Ag(CN)2- forms? Ksp for AgCl is 1.8 × 10-10 and Kf for Ag(CN)2- is 1.0 × 1021.
A)0.050 M
B)0.10 M
C)0.20 M
D)0.40 M
A)0.050 M
B)0.10 M
C)0.20 M
D)0.40 M

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
47
The dissociation equilibrium constants for the protonated form of alanine (a diprotic amino acid,H2X+)are Ka1 = 4.6 × 10-3 and Ka2 = 2.0 × 10-10.What is the pH of 50.00 mL of a 0.050 M solution of alanine after 37.50 mL of 0.100 M NaOH has been added?
A)4.85
B)6.02
C)7.39
D)9.70
A)4.85
B)6.02
C)7.39
D)9.70

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
48
Oxalic acid,H2C2O4 has acid dissociation constants Ka1 = 5.9 × 10-2 and Ka2 = 6.4 × 10-5.What is the pH after 20.00 mL of 0.0500 M NaOH is added to 5.00 mL of 0.2000 M H2C2O4?
A)1.23
B)2.10
C)2.80
D)4.19
A)1.23
B)2.10
C)2.80
D)4.19

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
49
What is the pH at the equivalence point of a weak base-strong acid titration if 20.00 mL of NaOCl requires 28.30 mL of 0.50 M HCl? Ka = 3.0 × 10-8 for HOCl.
A)0.30
B)3.18
C)3.76
D)4.03
A)0.30
B)3.18
C)3.76
D)4.03

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following reactions are not consistent with the concept of acid base amphoterism?
A)Al(OH)3(s)+ OH-(aq)→ Al(OH)4-(aq)
B)Al(OH)3(s)+ 3 H3O+(aq)→ Al3+(aq)+ 6 H2O(l)
C)H2O(l)+ H2O(l)⇌ H3O+(aq)+ OH-(aq)
D)Al(OH)3(s)⇌ Al3+(aq)+ 3 OH-(aq)
A)Al(OH)3(s)+ OH-(aq)→ Al(OH)4-(aq)
B)Al(OH)3(s)+ 3 H3O+(aq)→ Al3+(aq)+ 6 H2O(l)
C)H2O(l)+ H2O(l)⇌ H3O+(aq)+ OH-(aq)
D)Al(OH)3(s)⇌ Al3+(aq)+ 3 OH-(aq)

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
51
What is the molar solubility of CaF2 in 0.10 M NaF solution at 25°C? The Ksp for CaF2 is 3.4 × 10-11.
A)8.5 × 10-10 M
B)3.4 × 10-10 M
C)3.4 × 10-9 M
D)2.0 × 10-4 M
A)8.5 × 10-10 M
B)3.4 × 10-10 M
C)3.4 × 10-9 M
D)2.0 × 10-4 M

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
52
What is the molar solubility of AgCl in 1.0 M K2S2O3 if the complex ion Ag(S2O3)23- forms? The Ksp for AgCl is 1.8 × 10-10 and the Kf for Ag(S2O3)23- is 2.9 × 1013.
A)0.50 M
B)1.0 M
C)1.5 M
D)2.0 M
A)0.50 M
B)1.0 M
C)1.5 M
D)2.0 M

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
53
What is the molar solubility of Mg(OH)2 in a basic solution with a pH of 12.00? Ksp for Mg(OH)2 is 
A)5.6 × 10-10 M
B)5.6 × 10-8 M
C)2.4 × 10-6 M
D)1.1 × 10-4 M

A)5.6 × 10-10 M
B)5.6 × 10-8 M
C)2.4 × 10-6 M
D)1.1 × 10-4 M

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
54
Calculate the solubility (in g/L)of silver carbonate in water at 25°C if the Ksp for Ag2CO3 is 8.4 × 10-12.
A)8.0 × 10-4 g/L
B)3.5 × 10-2 g/L
C)4.4 × 10-2 g/L
D)5.6 × 10-2 g/L
A)8.0 × 10-4 g/L
B)3.5 × 10-2 g/L
C)4.4 × 10-2 g/L
D)5.6 × 10-2 g/L

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
55
Calculate the Ksp for silver sulfate if the solubility of Ag2SO4 in pure water is 4.5 g/L.
A)3.0 × 10-6
B)1.2 × 10-5
C)2.1 × 10-4
D)4.2 × 10-4
A)3.0 × 10-6
B)1.2 × 10-5
C)2.1 × 10-4
D)4.2 × 10-4

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
56
Sulfurous acid,H2SO3 has acid dissociation constants Ka1 = 1.5 × 10-2 and Ka2 = 6.3 × 10-8.What is the pH after 10.00 mL of 0.1000 M NaOH is added to 10.00 mL of 0.1000 M H2SO3?
A)1.82
B)3.60
C)4.25
D)7.20
A)1.82
B)3.60
C)4.25
D)7.20

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following metal hydroxides are amphoteric?
A)Al(OH)3,Zn(OH)2,Cr(OH)3,Sn(OH)2
B)Cu(OH)2,Mn(OH)2,Fe(OH)2,Fe(OH)3
C)Be(OH)2,Ca(OH)2,Ba(OH)2,Sr(OH)3
D)LiOH,NaOH,KOH,RbOH
A)Al(OH)3,Zn(OH)2,Cr(OH)3,Sn(OH)2
B)Cu(OH)2,Mn(OH)2,Fe(OH)2,Fe(OH)3
C)Be(OH)2,Ca(OH)2,Ba(OH)2,Sr(OH)3
D)LiOH,NaOH,KOH,RbOH

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
58
What is the pH of the resulting solution if 25 mL of 0.432 M methylamine,CH3NH2,is added to 15 mL of 0.234 M HCl? Assume that the volumes of the solutions are additive.Ka = 2.70 × 10-11 for CH3NH3+.
A)3.11
B)3.74
C)10.26
D)10.89
A)3.11
B)3.74
C)10.26
D)10.89

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
59
What is the molar solubility of lead(II)chromate in 0.10 M HNO3 if the Ksp for PbCrO4 is 2.8 × 10-13 and the Ka2 for H2CrO4 is 3.0 × 10-7? Note that H2CrO4 is considered to be a strong acid.
A)9.2 × 10-11 M
B)2.9 × 10-10 M
C)9.3 × 10-7 M
D)3.1 × 10-4 M
A)9.2 × 10-11 M
B)2.9 × 10-10 M
C)9.3 × 10-7 M
D)3.1 × 10-4 M

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
60
The dissociation equilibrium constants for the protonated form of alanine (a diprotic amino acid,H2X+)are Ka1 = 4.6 × 10-3 and Ka2 = 2.0 × 10-10.What is the pH of 50.00 mL of a 0.0500 M solution of alanine after 25.00 mL of 0.100 M NaOH has been added?
A)2.34
B)4.85
C)5.59
D)6.72
A)2.34
B)4.85
C)5.59
D)6.72

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
61
The following pictures represent solutions that contain a weak acid HA (pKa = 5.0)and its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 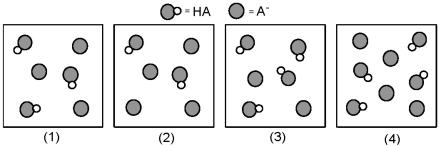
Which solution has the highest pH?
A)(1)
B)(2)
C)(3)
D)(4)

Which solution has the highest pH?
A)(1)
B)(2)
C)(3)
D)(4)

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
62
The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 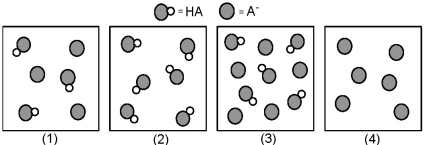
Which solution has the lowest pH?
A)(1)
B)(2)
C)(3)
D)(4)

Which solution has the lowest pH?
A)(1)
B)(2)
C)(3)
D)(4)

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
63
The following pictures represent solutions that contain a weak acid HA (pKa = 5.0)and its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 
Which of these solutions are buffers?
A)(1)and (2)
B)(1)and (3)
C)(1), (2)and (3)
D)All are buffer solutions.

Which of these solutions are buffers?
A)(1)and (2)
B)(1)and (3)
C)(1), (2)and (3)
D)All are buffer solutions.

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
64
A solution may contain the following ions Ag+,Cu2+,Mn2+,Ca2+,and Na+.No precipitate formed when 0.10 M NaCl was added but a dark colored precipitate formed when H2S was added to an acidic portion of the solution.After the removal of the solid the solution was made basic and more H2S was added and a dark precipitate again formed.Treatment of the filtrate with (NH4)2CO3 resulted in a white precipitate.If no further tests were made then what conclusions can you draw?
A)possible ions present Cu2+,Mn2+,Na+
B)possible ions present Cu2+,Mn2+,Ca2+
C)possible ions present Cu2+,Mn2+,Ca2+,Na+
D)possible ions present Ag+,Cu2+,Mn2+,Ca2+,Na+
A)possible ions present Cu2+,Mn2+,Na+
B)possible ions present Cu2+,Mn2+,Ca2+
C)possible ions present Cu2+,Mn2+,Ca2+,Na+
D)possible ions present Ag+,Cu2+,Mn2+,Ca2+,Na+

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
65
0.10 M potassium chromate is slowly added to a solution containing 0.20 M AgNO3 and 0.20 M Ba(NO3)2.What is the Ag+ concentration when BaCrO4 just starts to precipitate? Ksp for Ag2CrO4 and BaCrO4 are 1.1 × 10-12 and 1.2 × 10-10,respectively.
A)6.5 × 10-5 M
B)1.3 × 10-4 M
C)3.2 × 10-4 M
D)4.3 × 10-2 M
A)6.5 × 10-5 M
B)1.3 × 10-4 M
C)3.2 × 10-4 M
D)4.3 × 10-2 M

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
66
Which metal sulfides can be precipitated from a solution that is 0.01 M in Mn2+,Zn2+,Pb2+ and Cu2+ and 0.10 M in H2S at a pH of 0.50? 
A)MnS
B)CuS
C)PbS,CuS
D)ZnS,PbS,CuS

A)MnS
B)CuS
C)PbS,CuS
D)ZnS,PbS,CuS

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
67
The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 
Which of the solutions are buffer solutions?
A)(1)and (2)
B)(1)and (3)
C)(2)and (3)
D)(2)and (4)

Which of the solutions are buffer solutions?
A)(1)and (2)
B)(1)and (3)
C)(2)and (3)
D)(2)and (4)

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
68
The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 
Which solution has the greatest buffer capacity?
A)(1)
B)(2)
C)(3)
D)(4)

Which solution has the greatest buffer capacity?
A)(1)
B)(2)
C)(3)
D)(4)

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
69
The following pictures represent solutions that contain a weak acid HA (pKa = 5.0)and its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 
Which solution has the greatest buffer capacity?
A)(1)
B)(2)
C)(3)
D)(4)

Which solution has the greatest buffer capacity?
A)(1)
B)(2)
C)(3)
D)(4)

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
70
The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 
Which solution has the largest percent dissociation of HA?
A)(1)
B)(2)
C)(3)
D)(4)

Which solution has the largest percent dissociation of HA?
A)(1)
B)(2)
C)(3)
D)(4)

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
71
The following pictures represent solutions that contain a weak acid HA (pKa = 5.0)and its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 
Which solution has the largest percent dissociation of HA?
A)(1)
B)(2)
C)(3)
D)(4)

Which solution has the largest percent dissociation of HA?
A)(1)
B)(2)
C)(3)
D)(4)

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
72
The following pictures represent solutions that contain a weak acid HA (pKa = 5.0)and its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 
Which solution has the lowest pH?
A)(1)
B)(2)
C)(3)
D)(4)

Which solution has the lowest pH?
A)(1)
B)(2)
C)(3)
D)(4)

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
73
The following pictures represent solutions that contain a weak acid HA (pKa = 5.0)and its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 
For which of these solutions is pH = pKa?
A)All have pH = pKa.
B)(1), (2)and (3)
C)(1)and (4)
D)(2)and (3)

For which of these solutions is pH = pKa?
A)All have pH = pKa.
B)(1), (2)and (3)
C)(1)and (4)
D)(2)and (3)

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
74
Precipitation of an ionic compound will occur upon mixing of desired reagents if the initial ion product is
A)greater than the Ksp.
B)equal to the pKsp.
C)equal to the Ksp.
D)less than the Ksp.
A)greater than the Ksp.
B)equal to the pKsp.
C)equal to the Ksp.
D)less than the Ksp.

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
75
Potassium chromate is slowly added to a solution containing 0.20 M AgNO3 and 0.20 M Ba(NO3)2.Describe what happens if the Ksp for Ag2CrO4 is 1.1 × 10-12 and the Ksp of BaCrO4 is 1.2 × 10-10.
A)The BaCrO4 precipitates first out of solution.
B)The Ag2CrO4 precipitates first out of solution and then BaCrO4 precipitates.
C)Both BaCrO4 and Ag2CrO4 precipitate simultaneously out of solution.
D)Neither BaCrO4 nor Ag2CrO4 precipitates out of solution.
A)The BaCrO4 precipitates first out of solution.
B)The Ag2CrO4 precipitates first out of solution and then BaCrO4 precipitates.
C)Both BaCrO4 and Ag2CrO4 precipitate simultaneously out of solution.
D)Neither BaCrO4 nor Ag2CrO4 precipitates out of solution.

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
76
The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 
For which solution(s)is pH = pKa?
A)only solution (1)
B)only solution (2)
C)only solution (3)
D)solutions (1)and (3)

For which solution(s)is pH = pKa?
A)only solution (1)
B)only solution (2)
C)only solution (3)
D)solutions (1)and (3)

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
77
The following pictures represent solutions that contain a weak acid HA (pKa = 5.0)and its potassium salt KA.Unshaded spheres represent H atoms,black spheres represent oxygen atoms,and shaded spheres represent A- ions.(K+,H3O+ initially present,OH- initially present and solvent water molecules have been omitted for clarity. ) 
Which picture represents the equilibrium state of the solution after addition of one H3O+ ion to the solution shown in picture (1)?
A)(2)
B)(3)
C)(4)
D)(5)

Which picture represents the equilibrium state of the solution after addition of one H3O+ ion to the solution shown in picture (1)?
A)(2)
B)(3)
C)(4)
D)(5)

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
78
A solution may contain the following ions Ag+,Cu2+,Cd2+,Mn2+,Ni2+ and Na+.A white precipitate formed when 0.10 M NaCl was added and after this was removed the solution was treated with H2S gas under acidic conditions and no precipitate formed.When the solution was made basic and again treated with H2S gas a dark colored precipitate formed.If no further tests were made then what conclusions can you draw?
A)possible ions present Ag+,Mn2+,Ni2+
B)possible ions present Ag+,Mn2+,Ni2+,Na+
C)possible ions present Ag+,Cu2+,Cd2+
D)possible ions present Ag+,Cu2+,Cd2+,Na+
A)possible ions present Ag+,Mn2+,Ni2+
B)possible ions present Ag+,Mn2+,Ni2+,Na+
C)possible ions present Ag+,Cu2+,Cd2+
D)possible ions present Ag+,Cu2+,Cd2+,Na+

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
79
The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 
Which solution has the highest pH?
A)(1)
B)(2)
C)(3)
D)(4)

Which solution has the highest pH?
A)(1)
B)(2)
C)(3)
D)(4)

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
80
Which metal sulfides can be precipitated from a solution that is 0.01 M in Mn2+,Zn2+,Pb2+ and Cu2+ and 0.10 M in H2S at a pH of 1.0? 
A)MnS
B)CuS
C)PbS,CuS
D)ZnS,PbS,CuS

A)MnS
B)CuS
C)PbS,CuS
D)ZnS,PbS,CuS

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck


