Deck 20: Transition Elements and Coordination Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/190
Play
Full screen (f)
Deck 20: Transition Elements and Coordination Chemistry
1
What statement is most inconsistent with the chemistry of transition elements?
A)Bromide,chloride and iodide stabilize the higher oxidation states of the transition elements.
B)Early transition metal ions with the metal in its lowest oxidation state are good reducing agents.
C)Ions that have transition metal in their highest oxidation state tend to be good oxidizing agents.
D)The stability of the higher oxidation states increases down a periodic group.
A)Bromide,chloride and iodide stabilize the higher oxidation states of the transition elements.
B)Early transition metal ions with the metal in its lowest oxidation state are good reducing agents.
C)Ions that have transition metal in their highest oxidation state tend to be good oxidizing agents.
D)The stability of the higher oxidation states increases down a periodic group.
Bromide,chloride and iodide stabilize the higher oxidation states of the transition elements.
2
Transition series elements are all
A)gases.
B)metals.
C)nonmetals.
D)semimetals.
A)gases.
B)metals.
C)nonmetals.
D)semimetals.
metals.
3
Though we would expect an increase in atomic radii going down a group from the second to the third transition series of elements,the actual radii are nearly identical.The term commonly used to describe this phenomenon is the
A)atomic disparity.
B)effective nuclear charge.
C)lanthanide contraction.
D)transition default.
A)atomic disparity.
B)effective nuclear charge.
C)lanthanide contraction.
D)transition default.
lanthanide contraction.
4
Which transition metal has the anomalous ground-state electron configuration: [Kr] 4d10?
A)Rh
B)Pd
C)Ag
D)Cd
A)Rh
B)Pd
C)Ag
D)Cd

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
5
What is the strongest oxidizing agent of the following set: MnCl2,Mn(OH)3,MnO2,KMnO4?
A)MnCl2
B)Mn(OH)3
C)MnO2
D)KMnO4
A)MnCl2
B)Mn(OH)3
C)MnO2
D)KMnO4

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
6
Element M has the valence electron configuration 3d6 4s2.What is the valence electron configuration of the M3+ ion?
A)3d5
B)3d3 4s2
C)3d4 4s1
D)3d9 4s2
A)3d5
B)3d3 4s2
C)3d4 4s1
D)3d9 4s2

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
7
The number of transition series is
A)one
B)two
C)four
D)seven
A)one
B)two
C)four
D)seven

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
8
What is the d orbital-filling diagram for Fe3+ (Z = 26)? 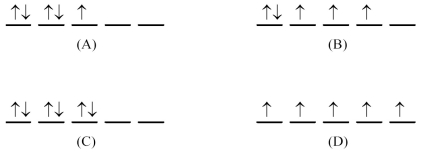
A)(A)
B)(B)
C)(C)
D)(D)

A)(A)
B)(B)
C)(C)
D)(D)

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
9
What is the ground-state electron configuration for the element chromium (Z = 24)?
A)[Ne] 4s2 3d4
B)[Ar] 4s2 3d4
C)[Ar] 4s1 3d5
D)[Ar] 3d6
A)[Ne] 4s2 3d4
B)[Ar] 4s2 3d4
C)[Ar] 4s1 3d5
D)[Ar] 3d6

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
10
What is the ground-state electron configuration for the element cobalt (Z = 27)?
A)[Ar] 4s2 3d7
B)[Ar] 3d9
C)[Ar] 4s2 4p6 5s1
D)[Ar] 3d7
A)[Ar] 4s2 3d7
B)[Ar] 3d9
C)[Ar] 4s2 4p6 5s1
D)[Ar] 3d7

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements best explains why transition metals lose electrons from s orbitals before d orbitals?
A)Electrons from s orbitals are lower in energy than d orbitals.
B)The d orbitals experience a greater drop in energy when electrons are removed.
C)Electrons from d orbitals are less shielded from the nucleus than electrons in s orbitals.
D)Electrons in s orbitals are paired.
A)Electrons from s orbitals are lower in energy than d orbitals.
B)The d orbitals experience a greater drop in energy when electrons are removed.
C)Electrons from d orbitals are less shielded from the nucleus than electrons in s orbitals.
D)Electrons in s orbitals are paired.

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
12
For transition elements,which of the following occurs as the effective nuclear charge increases?
A)The atomic radius increases.
B)The density increases.
C)Both the atomic radius and the density increase.
D)The atomic radius decreases and the density increases.
A)The atomic radius increases.
B)The density increases.
C)Both the atomic radius and the density increase.
D)The atomic radius decreases and the density increases.

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
13
What is the characteristic outer electron configuration for transition elements?
A)(n - 1)d10-xns2
B)(n)d10-xns2
C)(n + 1)d10-xns1
D)(n - 1)d10-x(n + 1)s2
A)(n - 1)d10-xns2
B)(n)d10-xns2
C)(n + 1)d10-xns1
D)(n - 1)d10-x(n + 1)s2

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
14
In which blocks of the periodic table are the transition series and inner transition series elements found?
A)d,p
B)d,f
C)s,d
D)s,p
A)d,p
B)d,f
C)s,d
D)s,p

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
15
What oxidation state(s)is(are)exhibited by all first row transition elements except scandium?
A)+2
B)+3
C)+2 and +3
D)+2,+3 and +4
A)+2
B)+3
C)+2 and +3
D)+2,+3 and +4

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
16
What is the ground-state electron configuration for Co2+ (Z = 27)?
A)[Ar] 4s2 3d9
B)[Ar] 4s2 3d5
C)[Ar] 3d7
D)[Ar] 4s1 3d6
A)[Ar] 4s2 3d9
B)[Ar] 4s2 3d5
C)[Ar] 3d7
D)[Ar] 4s1 3d6

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following ions has the greater effective nuclear charge?
A)V3+
B)Cr3+
C)Fe3+
D)Co3+
A)V3+
B)Cr3+
C)Fe3+
D)Co3+

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
18
What is the ground-state electron configuration for Cr in Cr2O72-?
A)[Ar] 4s1 3d5
B)[Ar] 4s2 3d6
C)[Ar] 3d4
D)[Ar] 3d0
A)[Ar] 4s1 3d5
B)[Ar] 4s2 3d6
C)[Ar] 3d4
D)[Ar] 3d0

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
19
How many d electrons are there in MnO4-?
A)0
B)1
C)2
D)3
A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
20
What is the strongest oxidizing agent of the following set: VCl2,CrCl3,KMnO4,KReO4?
A)VCl2
B)CrCl3
C)KMnO4
D)KReO4
A)VCl2
B)CrCl3
C)KMnO4
D)KReO4

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
21
Which is the strongest oxidizing agent under acidic conditions?
A)Cr2+
B)Cr3+
C)CrO42-
D)Cr2O72-
A)Cr2+
B)Cr3+
C)CrO42-
D)Cr2O72-

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following ions should be the strongest reducing agent?
A)V3+
B)Cr3+
C)Fe3+
D)Co3+
A)V3+
B)Cr3+
C)Fe3+
D)Co3+

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is a disproportionation reaction?
A)Cu2S(l)+ O2(g)→ 2 Cu(l)+ SO2(g)
B)3 Cu(s)+ 2 NO3-(aq)+ 8 H+(aq)→ 3 Cu2+(aq)+ 2 NO(g)+ 4 H2O(l)
C)2 Cu+(aq)→ Cu(s)+ Cu2+(aq)
D)Cu2+(aq)+ 4 NH3(aq)→ Cu(NH3)42+(aq)
A)Cu2S(l)+ O2(g)→ 2 Cu(l)+ SO2(g)
B)3 Cu(s)+ 2 NO3-(aq)+ 8 H+(aq)→ 3 Cu2+(aq)+ 2 NO(g)+ 4 H2O(l)
C)2 Cu+(aq)→ Cu(s)+ Cu2+(aq)
D)Cu2+(aq)+ 4 NH3(aq)→ Cu(NH3)42+(aq)

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is a stronger acid?
A)Cr3+
B)Cr(OH)2
C)Cr(OH)3
D)CrO2(OH)2
A)Cr3+
B)Cr(OH)2
C)Cr(OH)3
D)CrO2(OH)2

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
25
What chemical equation represents the best method for obtaining pure chromium?
A)FeCr2O4(s)+ 4 C(s)+ heat → Fe(s)+ 2 Cr(s)+ 4 CO(g)
B)Cr2O3(s)+ 2 Al(s)+ heat → 2 Cr(s)+ Al2O3(s)
C)Cr2O3(s)+ 2 Fe(s)+ heat → Fe2O3(s)+ 2 Cr(s)
D)Cr2+(aq)+ H2(g)→ Cr(s)+ 2 H+(aq)
A)FeCr2O4(s)+ 4 C(s)+ heat → Fe(s)+ 2 Cr(s)+ 4 CO(g)
B)Cr2O3(s)+ 2 Al(s)+ heat → 2 Cr(s)+ Al2O3(s)
C)Cr2O3(s)+ 2 Fe(s)+ heat → Fe2O3(s)+ 2 Cr(s)
D)Cr2+(aq)+ H2(g)→ Cr(s)+ 2 H+(aq)

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following chromium species is the strongest acid?
A)Cr(OH)2
B)Cr(OH)3
C)CrO2(OH)2
D)CrO42-
A)Cr(OH)2
B)Cr(OH)3
C)CrO2(OH)2
D)CrO42-

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
27
What is the oxidation state of the Co atom in [Co(NH3)5Cl](NO3)2?
A)+2
B)+3
C)+4
D)+6
A)+2
B)+3
C)+4
D)+6

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
28
What is the oxidation state of the Cr atom in [Ni(en)3]3[Cr(CN)6]2?
A)+2
B)+3
C)+4
D)+6
A)+2
B)+3
C)+4
D)+6

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
29
Describe what happens when 3.0 M NH3 is slowly added to an aqueous solution of CuSO4.
A)A blue precipitate of Cu(OH)2 forms.
B)A royal blue complex of [Cu(NH3)4]2+ is formed.
C)A blue precipitate of Cu(OH)2 is formed which is then converted to the royal blue complex [Cu(NH3)4]2+.
D)A royal blue complex of [Cu(NH3)4]2+ is formed which then is converted to a blue precipitate of Cu(OH)2.
A)A blue precipitate of Cu(OH)2 forms.
B)A royal blue complex of [Cu(NH3)4]2+ is formed.
C)A blue precipitate of Cu(OH)2 is formed which is then converted to the royal blue complex [Cu(NH3)4]2+.
D)A royal blue complex of [Cu(NH3)4]2+ is formed which then is converted to a blue precipitate of Cu(OH)2.

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
30
What statement is most inconsistent about the chemistry of iron?
A)Iron(III)hydroxide is very soluble and reacts readily with hydroxide to form Fe(OH)4-.
B)Iron reacts with hydrochloric acid in the absence of air to yield iron(II)ion and hydrogen gas.
C)Iron reacts with nitric acid to yield iron(III)ion and nitric oxide.
D)The most common oxidation states of iron are +2 (ferrous)and +3 (ferric).
A)Iron(III)hydroxide is very soluble and reacts readily with hydroxide to form Fe(OH)4-.
B)Iron reacts with hydrochloric acid in the absence of air to yield iron(II)ion and hydrogen gas.
C)Iron reacts with nitric acid to yield iron(III)ion and nitric oxide.
D)The most common oxidation states of iron are +2 (ferrous)and +3 (ferric).

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
31
What is the coordination number of the Fe atom in K3[Fe(C2O4)3]?
A)2
B)3
C)4
D)6
A)2
B)3
C)4
D)6

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
32
What is the strongest oxidizing agent of the following set: FeO,Fe2O3,Fe3O4,FeO42-?
A)FeO
B)Fe2O3
C)Fe3O4
D)FeO42-
A)FeO
B)Fe2O3
C)Fe3O4
D)FeO42-

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
33
What statement is inconsistent with the chemistry of iron?
A)Iron is the fourth most abundant element in the earth's crust.
B)Iron is obtained from the reduction of hematite (Fe2O3)and magnetite (Fe3O4)by carbon in a blast furnace.
C)Iron is a relatively hard metal and it is relatively unreactive with haloacids.
D)The majority of iron in a healthy human is present in the oxygen-carrying protein hemoglobin.
A)Iron is the fourth most abundant element in the earth's crust.
B)Iron is obtained from the reduction of hematite (Fe2O3)and magnetite (Fe3O4)by carbon in a blast furnace.
C)Iron is a relatively hard metal and it is relatively unreactive with haloacids.
D)The majority of iron in a healthy human is present in the oxygen-carrying protein hemoglobin.

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
34
Which is not a characteristic reaction of chromium metal or chromium(II)ion?
A)Cr(s)+ 2 H+(aq)→ Cr2+(aq)+ H2(g)
B)4 Cr2+(aq)+ O2(g)+ 4 H+(aq)→ 4 Cr3+(aq)+ 2 H2O(l)
C)Cr(OH)2(s)+ 2 H3O+(aq)→ Cr2+(aq)+ 4 H2O(l)
D)Cr(OH)2(s)+ OH-(aq)→ Cr(OH)3-(aq)
A)Cr(s)+ 2 H+(aq)→ Cr2+(aq)+ H2(g)
B)4 Cr2+(aq)+ O2(g)+ 4 H+(aq)→ 4 Cr3+(aq)+ 2 H2O(l)
C)Cr(OH)2(s)+ 2 H3O+(aq)→ Cr2+(aq)+ 4 H2O(l)
D)Cr(OH)2(s)+ OH-(aq)→ Cr(OH)3-(aq)

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
35
In the two half-reactions shown below,which chromium species is the strongest oxidizing agent? Cr2O72-(aq)+ 14 H+(aq)+ 6 e- → 2 Cr3+(aq)+ 7 H2O(l)E° = + 1.33 V
CrO42-(aq)+ 4 H2O(l)+ 6 e- → Cr(OH)3(s)+ 5 OH-(aq)E° = - 0.13 V
A)Cr2O72-
B)Cr3+
C)CrO42-
D)Cr(OH)3
CrO42-(aq)+ 4 H2O(l)+ 6 e- → Cr(OH)3(s)+ 5 OH-(aq)E° = - 0.13 V
A)Cr2O72-
B)Cr3+
C)CrO42-
D)Cr(OH)3

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
36
What is the coordination number of the Au atom in K [Au(CN)2(SCN)2]?
A)2
B)3
C)4
D)6
A)2
B)3
C)4
D)6

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
37
What statement is inconsistent with the chemistry of copper?
A)It has a high electrical conductivity and is widely used to make electrical wiring.
B)It is commonly found in the elemental state.
C)It is a reddish colored metal that accounts for only 0.0068% of the earth's crust by mass.
D)It is used to make corrosion-resistant water pipes because it has a positive oxidation potential.
A)It has a high electrical conductivity and is widely used to make electrical wiring.
B)It is commonly found in the elemental state.
C)It is a reddish colored metal that accounts for only 0.0068% of the earth's crust by mass.
D)It is used to make corrosion-resistant water pipes because it has a positive oxidation potential.

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
38
Using the following reduction potentials for copper determine the unstable copper compound. Cu+(aq)+ e- → Cu(s)E° = +0.52 V
Cu2+(aq)+ e- → Cu+(aq)E° = +0.15 V
A)CuCl
B)CuCl2
C)CuSO4
D)Cu(OH)2
Cu2+(aq)+ e- → Cu+(aq)E° = +0.15 V
A)CuCl
B)CuCl2
C)CuSO4
D)Cu(OH)2

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
39
Which chromium species exists only under acidic conditions?
A)Cr(OH)2
B)Cr(OH)4-
C)CrO42-
D)Cr2O72-
A)Cr(OH)2
B)Cr(OH)4-
C)CrO42-
D)Cr2O72-

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
40
What are two of the major components of stainless steel?
A)iron and carbon
B)iron and chromium
C)iron and titanium
D)iron and tungsten
A)iron and carbon
B)iron and chromium
C)iron and titanium
D)iron and tungsten

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
41
Which metal ion is most likely to form a square planar complex ion with CN-?
A)Co2+
B)Cu2+
C)Ni2+
D)Zn2+
A)Co2+
B)Cu2+
C)Ni2+
D)Zn2+

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
42
The complex [Ni(CN)4]2- is diamagnetic and the complex [NiCl4]2- is paramagnetic.What can you conclude about their molecular geometries?
A)Both complexes have square planar geometries.
B)Both complexes have tetrahedral geometries.
C)[NiCl4]2- has a square planar geometry while [Ni(CN)4]2- has a tetrahedral geometry.
D)[NiCl4]2- has a tetrahedral geometry while [Ni(CN)4]2- has a square planar geometry.
A)Both complexes have square planar geometries.
B)Both complexes have tetrahedral geometries.
C)[NiCl4]2- has a square planar geometry while [Ni(CN)4]2- has a tetrahedral geometry.
D)[NiCl4]2- has a tetrahedral geometry while [Ni(CN)4]2- has a square planar geometry.

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
43
Write the chemical formula for aquabromobis(ethylenediamine)chromium(III)chloride.
A)[CrBr(H2O)(en)]Cl
B)[CrBr2(H2O)(en)]Cl2
C)[CrBr(H2O)(en)2]Cl2
D)[CrBr(H2O)(en)2]Cl3
A)[CrBr(H2O)(en)]Cl
B)[CrBr2(H2O)(en)]Cl2
C)[CrBr(H2O)(en)2]Cl2
D)[CrBr(H2O)(en)2]Cl3

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
44
What is the name of the complex [Ni(H2O)4(NH2CH2CH2NH2)]SO4 ∙ 5H2O?
A)aquaethylenediaminenickel(II)sulfate hydrate
B)tetraaquaethylenediaminenickel(II)sulfate pentahydrate
C)tetraaquabis(ethylenediamine)nickel(II)sulfate pentahydrate
D)tetraaquabis(ethylenediamine)nickel(III)sulfate pentahydrate
A)aquaethylenediaminenickel(II)sulfate hydrate
B)tetraaquaethylenediaminenickel(II)sulfate pentahydrate
C)tetraaquabis(ethylenediamine)nickel(II)sulfate pentahydrate
D)tetraaquabis(ethylenediamine)nickel(III)sulfate pentahydrate

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
45
What hybridization scheme is used for Ni in the square planar complex of [Ni(CN)4]2-?
A)sp3
B)dsp2
C)dsp3
D)d2sp3
A)sp3
B)dsp2
C)dsp3
D)d2sp3

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
46
What is a representative orbital-filling diagram for the cobalt ion in the low spin complex of [Co(CN)6]3-? ![<strong>What is a representative orbital-filling diagram for the cobalt ion in the low spin complex of [Co(CN)<sub>6</sub>]<sup>3-</sup>? </strong> A)(A) B)(B) C)(C) D)(D)](https://storage.examlex.com/TB4940/11ea7e2d_d1c8_fe74_a2f7_455c0019f72a_TB4940_00.jpg)
A)(A)
B)(B)
C)(C)
D)(D)
![<strong>What is a representative orbital-filling diagram for the cobalt ion in the low spin complex of [Co(CN)<sub>6</sub>]<sup>3-</sup>? </strong> A)(A) B)(B) C)(C) D)(D)](https://storage.examlex.com/TB4940/11ea7e2d_d1c8_fe74_a2f7_455c0019f72a_TB4940_00.jpg)
A)(A)
B)(B)
C)(C)
D)(D)

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
47
What type of hybrid orbitals are used by the Ti atom to form chemical bonds in the complex ion [Ti(H2O)6]3+?
A)sp3
B)dsp2
C)dsp3
D)d2sp3
A)sp3
B)dsp2
C)dsp3
D)d2sp3

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
48
What is the name of the complex [Ni(en)3]3[Cr(CN)6]2?
A)ethylenediaminenickel(III)hexacyanochromate(II)
B)tris(ethylenediamine)nickel(III)hexacyanochromate(II)
C)tris(ethylenediamine)nickel(II)hexacyanochromate(III)
D)bis(ethylenediamine)nickel(II)hexacyanochromate(III)
A)ethylenediaminenickel(III)hexacyanochromate(II)
B)tris(ethylenediamine)nickel(III)hexacyanochromate(II)
C)tris(ethylenediamine)nickel(II)hexacyanochromate(III)
D)bis(ethylenediamine)nickel(II)hexacyanochromate(III)

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
49
Which complex is optically active?
A)[CoCl4en]2-
B)trans-[CrCl2(en)2]+
C)cis-[CrCl2(en)2]+
D)[PtCl2(NH3)2]
A)[CoCl4en]2-
B)trans-[CrCl2(en)2]+
C)cis-[CrCl2(en)2]+
D)[PtCl2(NH3)2]

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
50
The complex cis-[CoCl(NH3)(NH2CH2CH2NH2)2]2+ was resolved into optical isomers in 1911 by Alfred Werner,demonstrating the octahedral geometry of the ion.Name this complex ion.
A)cis-chloroammineethylenediaminecobalt(II)ion
B)cis-amminechloroethylenediaminecobalt(III)ion
C)cis-amminechlorobis(ethylenediamine)cobalt(II)ion
D)cis-amminechlorobis(ethylenediamine)cobalt(III)ion
A)cis-chloroammineethylenediaminecobalt(II)ion
B)cis-amminechloroethylenediaminecobalt(III)ion
C)cis-amminechlorobis(ethylenediamine)cobalt(II)ion
D)cis-amminechlorobis(ethylenediamine)cobalt(III)ion

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
51
How many unpaired electrons are present in the high spin form of the [CoF6]3- complex and what metal orbitals are used in bonding?
A)0 unpaired electrons and 4s,4p and 4d orbitals to give sp3d2
B)4 unpaired electrons and 4s,4p and 4d orbitals to give sp3d2
C)0 unpaired electrons and 3d,4s,and 4p orbitals to give d2sp3
D)4 unpaired electrons and 3d,4s,and 4p orbitals to give d2sp3
A)0 unpaired electrons and 4s,4p and 4d orbitals to give sp3d2
B)4 unpaired electrons and 4s,4p and 4d orbitals to give sp3d2
C)0 unpaired electrons and 3d,4s,and 4p orbitals to give d2sp3
D)4 unpaired electrons and 3d,4s,and 4p orbitals to give d2sp3

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
52
Write the chemical formula for pentaamminenitritocobalt(III)ion.
A)[Co(NO)(NH3)5]3+
B)[Co(NO2)(NH3)5]2+
C)[Co(ONO)(NH3)5]2+
D)[Co(NH3)5(N2O)]2+
A)[Co(NO)(NH3)5]3+
B)[Co(NO2)(NH3)5]2+
C)[Co(ONO)(NH3)5]2+
D)[Co(NH3)5(N2O)]2+

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
53
When the oxalate ion,C2O42- is bonded to the iron(III)ion in the complex ion [Fe(C2O4)3]3-,a ________-membered chelate ring is formed.
A)3
B)4
C)5
D)6
A)3
B)4
C)5
D)6

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
54
Which pair of isomers illustrates the concept of ionization isomers?
A)[Cr(SCN)(NH3)5]2+ and [Cr(NCS)(NH3)5]2+
B)[CoCl(NH3)5]SO4 and [Co(SO4)(NH3)5]Cl
C)cis -[PtCl2(NH3)2] and trans -[PtCl2(NH3)2]
D)(+)-[Co(en)3]3+ and (-)-[Co(en)3]3+
A)[Cr(SCN)(NH3)5]2+ and [Cr(NCS)(NH3)5]2+
B)[CoCl(NH3)5]SO4 and [Co(SO4)(NH3)5]Cl
C)cis -[PtCl2(NH3)2] and trans -[PtCl2(NH3)2]
D)(+)-[Co(en)3]3+ and (-)-[Co(en)3]3+

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following can function as a chelating agent?
A)CN-
B)CO
C)H2NCH2CH2NH2
D)NCS-
A)CN-
B)CO
C)H2NCH2CH2NH2
D)NCS-

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
56
What is the name of the complex ion [AuBrCl(CN)2]-?
A)bromochlorodicyanogold(I)ion
B)bromochlorodicyanoaurate(III)ion
C)bromochlorodicyanoargentate(III)ion
D)bromochlorodicyanoaurate(IV)ion
A)bromochlorodicyanogold(I)ion
B)bromochlorodicyanoaurate(III)ion
C)bromochlorodicyanoargentate(III)ion
D)bromochlorodicyanoaurate(IV)ion

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
57
The compounds [Cr(H2O)6]Cl3 and [CrCl3(H2O)3] ∙ 3H2O are examples of
A)diastereoisomers.
B)enantiomers.
C)ionization isomers.
D)linkage isomers.
A)diastereoisomers.
B)enantiomers.
C)ionization isomers.
D)linkage isomers.

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
58
A chromium(III)ion forms a complex ion with two ammonia molecules and four thiocyanate ions.What is the formula of the complex ion?
A)[Cr(NH3)2(NCS)4]3+
B)[Cr(NH4)2(NCS)4]+
C)[Cr(NH3)2(NCS)4]-
D)[Cr(NH3)2(NCS)4]4-
A)[Cr(NH3)2(NCS)4]3+
B)[Cr(NH4)2(NCS)4]+
C)[Cr(NH3)2(NCS)4]-
D)[Cr(NH3)2(NCS)4]4-

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following species is diamagnetic?
A)an isolated,gas-phase V3+ ion
B)a high-spin octahedral Fe2+ complex
C)an isolated,gas-phase Cu2+ ion
D)a low-spin octahedral Co3+ complex
A)an isolated,gas-phase V3+ ion
B)a high-spin octahedral Fe2+ complex
C)an isolated,gas-phase Cu2+ ion
D)a low-spin octahedral Co3+ complex

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
60
What is the correct formula for tetraamminecarbonatoiron(III)chloride?
A)(NH3)4[FeCO3]Cl
B)[Fe(CO3)(NH3)4]Cl
C)[Fe(CO3)(NH3)4]Cl2
D)[Fe(CO3)Cl(NH3)4]
A)(NH3)4[FeCO3]Cl
B)[Fe(CO3)(NH3)4]Cl
C)[Fe(CO3)(NH3)4]Cl2
D)[Fe(CO3)Cl(NH3)4]

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
61
How many unpaired electrons will Co have in the complex [CoCl4]2-?
A)1
B)3
C)4
D)5
A)1
B)3
C)4
D)5

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
62
What is the crystal field energy level diagram for the complex [Fe(H2O)6]3+? ![<strong>What is the crystal field energy level diagram for the complex [Fe(H<sub>2</sub>O)<sub>6</sub>]<sup>3+</sup>? </strong> A)(A) B)(B) C)(C) D)(D)](https://storage.examlex.com/TB4940/11ea7e2d_d1c9_4c95_a2f7_19eda1c5c43b_TB4940_00.jpg)
A)(A)
B)(B)
C)(C)
D)(D)
![<strong>What is the crystal field energy level diagram for the complex [Fe(H<sub>2</sub>O)<sub>6</sub>]<sup>3+</sup>? </strong> A)(A) B)(B) C)(C) D)(D)](https://storage.examlex.com/TB4940/11ea7e2d_d1c9_4c95_a2f7_19eda1c5c43b_TB4940_00.jpg)
A)(A)
B)(B)
C)(C)
D)(D)

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following complex ions is colorless?
A)[Co(H2O)6]2+
B)[Mn(CN)6]3-
C)[CrCl3(H2O)3]
D)[Ag(NH3)2]+
A)[Co(H2O)6]2+
B)[Mn(CN)6]3-
C)[CrCl3(H2O)3]
D)[Ag(NH3)2]+

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
64
![<strong> Which element indicated on the above periodic table has the electron configuration [Kr] 4d<sup>5</sup> 5s<sup>2</sup>?</strong> A)element A B)element B C)element C D)element D](https://storage.examlex.com/TB4940/11ea7e2d_d1c9_c1c8_a2f7_dda595d65e49_TB4940_00_TB4940_00_TB4940_00_TB4940_00_TB4940_00_TB4940_00.jpg)
Which element indicated on the above periodic table has the electron configuration [Kr] 4d5 5s2?
A)element A
B)element B
C)element C
D)element D

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
65
![<strong> Which element indicated on the above periodic table has the electron configuration [Xe] 4f<sup>7</sup> 5d<sup>1</sup> 6s<sup>2</sup>?</strong> A)element A B)element B C)element C D)element D](https://storage.examlex.com/TB4940/11ea7e2d_d1c9_c1c8_a2f7_dda595d65e49_TB4940_00_TB4940_00_TB4940_00_TB4940_00_TB4940_00_TB4940_00.jpg)
Which element indicated on the above periodic table has the electron configuration [Xe] 4f7 5d1 6s2?
A)element A
B)element B
C)element C
D)element D

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
66
The first transition series metals are shown below. 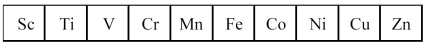
Which has the lowest melting point?
A)Sc
B)V
C)Mn
D)Zn

Which has the lowest melting point?
A)Sc
B)V
C)Mn
D)Zn

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
67
![<strong> Which element indicated on the above periodic table has the electron configuration [Ar] 3d<sup>10</sup> 4s<sup>1</sup>?</strong> A)element A B)element B C)element C D)element D](https://storage.examlex.com/TB4940/11ea7e2d_d1c9_c1c8_a2f7_dda595d65e49_TB4940_00_TB4940_00_TB4940_00_TB4940_00_TB4940_00_TB4940_00.jpg)
Which element indicated on the above periodic table has the electron configuration [Ar] 3d10 4s1?
A)element A
B)element B
C)element C
D)element D

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
68
Which ion would you expect to have the largest crystal field splitting Δ?
A)[Fe(CN)6]4-
B)[Fe(CN)6]3-
C)[Fe(H2O)6]2+
D)[Fe(H2O)6]3+
A)[Fe(CN)6]4-
B)[Fe(CN)6]3-
C)[Fe(H2O)6]2+
D)[Fe(H2O)6]3+

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
69
For an octahedral complex what metal d orbitals are directly towards the ligands?
A)dxy,dxz
B)dxy,dxz,dyz
C)dz²,dx²-y²
D)dz²,dxz,dyz
A)dxy,dxz
B)dxy,dxz,dyz
C)dz²,dx²-y²
D)dz²,dxz,dyz

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following complexes are diamagnetic? [Mn(CN)6]3- [Zn(NH3)4]2+ [Fe(CN)6]4- [FeF6]3-
A)[Zn(NH3)4]2+
B)[Zn(NH3)4]2+ and [FeF6]3-
C)[Zn(NH3)4]2+ and [Fe(CN)6]4-
D)[Mn(CN)6]3-,[Zn(NH3)4]2+ and [Fe(CN)6]4-
A)[Zn(NH3)4]2+
B)[Zn(NH3)4]2+ and [FeF6]3-
C)[Zn(NH3)4]2+ and [Fe(CN)6]4-
D)[Mn(CN)6]3-,[Zn(NH3)4]2+ and [Fe(CN)6]4-

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
71
What is the crystal field energy level diagram for the complex [Co(CN)6]3-? ![<strong>What is the crystal field energy level diagram for the complex [Co(CN)<sub>6</sub>]<sup>3-</sup>? </strong> A)(A) B)(B) C)(C) D)(D)](https://storage.examlex.com/TB4940/11ea7e2d_d1c9_73a6_a2f7_d5381e631ad4_TB4940_00.jpg)
A)(A)
B)(B)
C)(C)
D)(D)
![<strong>What is the crystal field energy level diagram for the complex [Co(CN)<sub>6</sub>]<sup>3-</sup>? </strong> A)(A) B)(B) C)(C) D)(D)](https://storage.examlex.com/TB4940/11ea7e2d_d1c9_73a6_a2f7_d5381e631ad4_TB4940_00.jpg)
A)(A)
B)(B)
C)(C)
D)(D)

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
72
What is the expected order for increasing octahedral (Δ0)crystal field splitting for ligands?
A)halides < O ligands < N ligands < CN-
B)halides < CN- < O ligands < N ligands
C)CN- < N ligands < O ligands < halides
D)O ligands < N ligands < CN- < halides
A)halides < O ligands < N ligands < CN-
B)halides < CN- < O ligands < N ligands
C)CN- < N ligands < O ligands < halides
D)O ligands < N ligands < CN- < halides

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
73
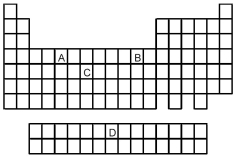
Which is the most common ion formed by element B on the above periodic table?
A)B+
B)B2+
C)B3+
D)B4+

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
74
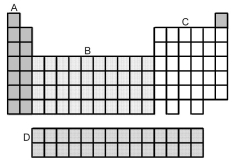
Which group of elements,indicated by letter and shading on the periodic table above,represents the inner transition elements?
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
75
![<strong> Which element indicated on the above periodic table has the electron configuration [Ar] 3d<sup>3</sup> 4s<sup>2</sup>?</strong> A)element A B)element B C)element C D)element D](https://storage.examlex.com/TB4940/11ea7e2d_d1c9_c1c8_a2f7_dda595d65e49_TB4940_00_TB4940_00_TB4940_00_TB4940_00_TB4940_00_TB4940_00.jpg)
Which element indicated on the above periodic table has the electron configuration [Ar] 3d3 4s2?
A)element A
B)element B
C)element C
D)element D

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
76

Which group of elements,indicated by letter and shading on the periodic table above,represents the transition elements?
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
77
What statement is inconsistent with the crystal field theory of tetrahedral complexes?
A)Δt is about 4/9 that of Δ0.
B)Tetrahedral complexes are nearly all low spin.
C)The dxy,dxz,and dyz orbitals are higher in energy than the dz² and dx²-y² orbitals.
D)None of the metal d orbitals point directly at the ligands in a tetrahedral environment.
A)Δt is about 4/9 that of Δ0.
B)Tetrahedral complexes are nearly all low spin.
C)The dxy,dxz,and dyz orbitals are higher in energy than the dz² and dx²-y² orbitals.
D)None of the metal d orbitals point directly at the ligands in a tetrahedral environment.

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
78
The first transition series metals are shown below. 
Which has the highest melting point?
A)Sc
B)V
C)Mn
D)Zn

Which has the highest melting point?
A)Sc
B)V
C)Mn
D)Zn

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
79

Which is the most common ion formed by element D on the above periodic table?
A)B+
B)B2+
C)B3+
D)B7+

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
80
What is the expected order for increasing octahedral (Δ0)crystal field splitting for the ligands I-,F-,H2O,NH3,en,CO?
A)I- < F- < H2O < NH3 < en < CO
B)F- < I- < NH3 < en < CO < H2O
C)I- < F- < H2O < CO < NH3 < en
D)CO < en < NH3 < H2O < F- < I-
A)I- < F- < H2O < NH3 < en < CO
B)F- < I- < NH3 < en < CO < H2O
C)I- < F- < H2O < CO < NH3 < en
D)CO < en < NH3 < H2O < F- < I-

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck


